


This article serves as a comprehensive step-by-step guide for mastering the FDA device listing and registration process, a crucial element for ensuring compliance and market access for medical devices in the United States. It delineates essential steps, highlights common pitfalls to avoid, and outlines ongoing responsibilities following registration. The emphasis on accurate classification, complete documentation, and timely fee payments is vital for facilitating successful regulatory compliance, thereby reinforcing the importance of a meticulous approach in navigating this complex landscape.
Navigating the intricate landscape of FDA device registration and listing is crucial for any medical device manufacturer aiming to bring their product to market. This process not only ensures compliance with regulatory standards but also safeguards public health by maintaining oversight of medical devices available to consumers. As the industry evolves and the stakes rise, understanding the nuances of this registration process becomes increasingly vital.
What common pitfalls could jeopardize a device's market entry?
How can manufacturers effectively streamline their registration efforts to avoid costly setbacks?
The FDA device listing and registration of instruments are essential components of the regulatory framework governing medical products in the United States. This process not only informs the FDA of the manufacturing establishment's location but also specifies the particular items produced at that site. Such duality is crucial for the FDA to maintain a comprehensive overview of all medical devices on the market, which is vital for ongoing safety and efficacy monitoring.
Ana Criado, our Director of Compliance Affairs, brings extensive expertise in this area, having held various leadership roles at Colombia’s oversight agency, INVIMA. Her background as a biomedical engineering educator and external regulatory advisor for international firms underscores the importance of meticulous adherence to listing requirements.
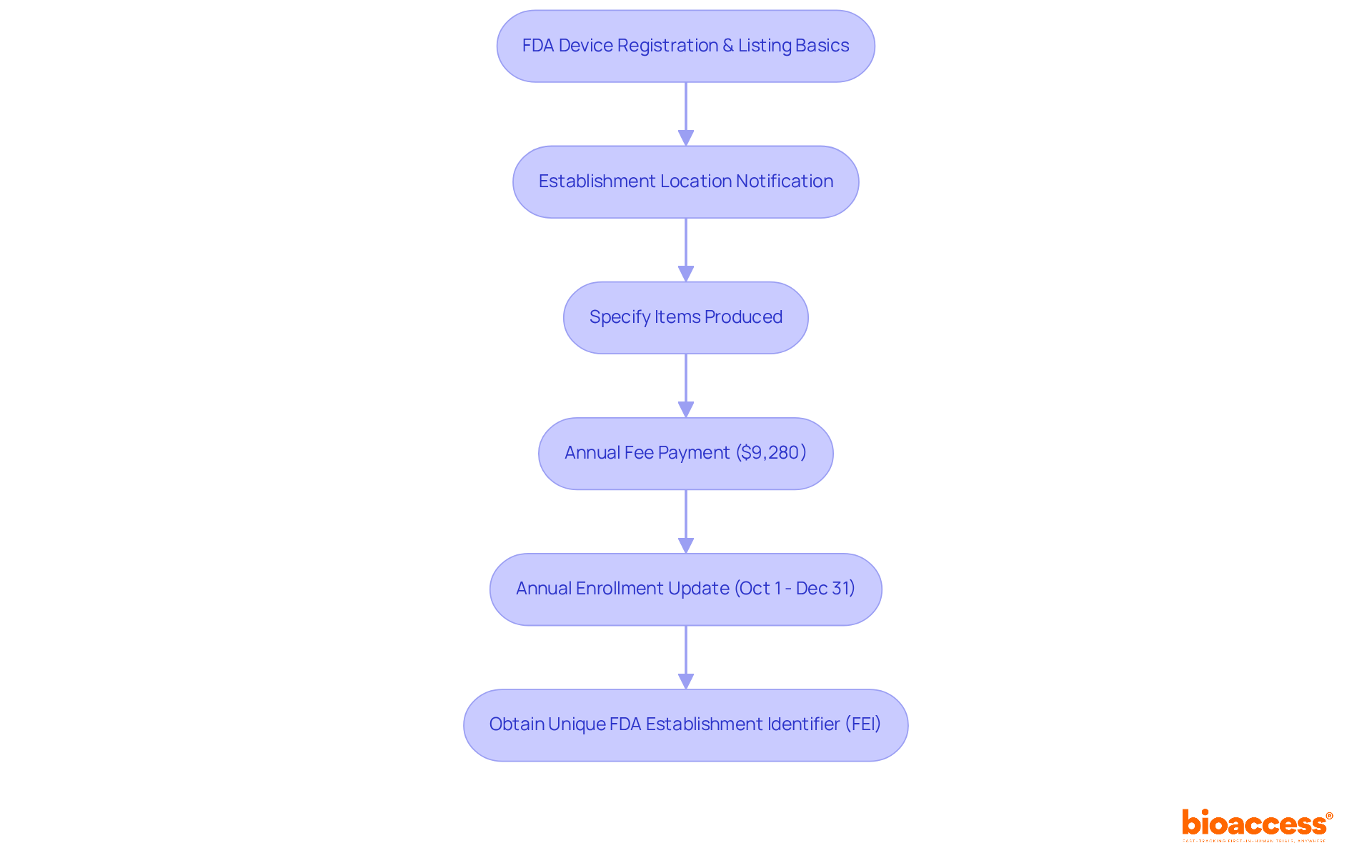
In the fiscal year 2025, the annual fee for FDA Device Facility enrollment is set at $9,280, emphasizing the financial commitment required for compliance. Furthermore, establishments must update their enrollment and listing annually between October 1 and December 31, ensuring that the FDA's records remain current and precise.
For the year ending September 2022, approximately 85 percent of 510(k) applications received a Substantially Equivalent decision, which indicates regulatory approval for market sale in the U.S. However, 15 percent of submissions did not achieve this status, highlighting the critical nature of adhering to listing requirements to avoid potential market access issues.
Understanding these fundamental concepts equips you for the subsequent stages in the enrollment process, including the necessity for a unique FDA Establishment Identifier (FEI) assigned during enrollment. This identifier is vital for effectively tracking and managing compliance. Familiarizing yourself with the FDA device listing and registration process is the initial step toward ensuring compliance with FDA regulations, ultimately facilitating smoother market entry for your medical products.
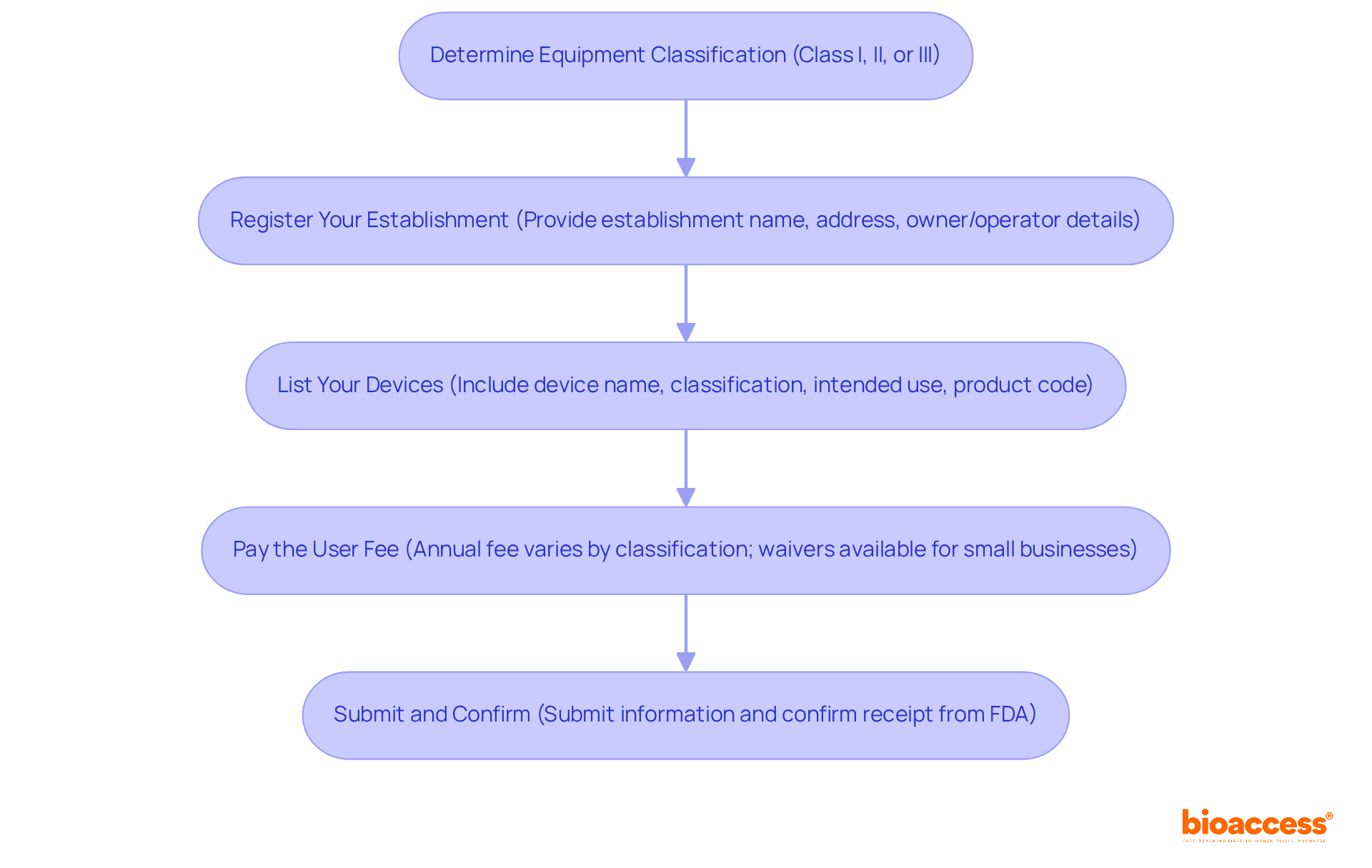
To successfully register your medical device with the FDA, follow these steps:
Determine Equipment Classification: Ascertain whether your equipment falls under Class I, II, or III, as this classification influences the compliance pathway you must follow.
To comply with regulations, manufacturers must ensure their products undergo FDA device listing and registration. Register your establishment by utilizing the FDA device listing and registration to register your manufacturing facility. Required information includes:
List Your Devices for FDA Device Listing and Registration: After registering your establishment, ensure you complete the FDA device listing and registration for all devices manufactured at that location. Include:
Pay the User Fee: Ensure payment of the necessary annual enrollment fee, which varies based on your establishment's classification. Small enterprises with gross receipts of no more than $1 million may be eligible for a waiver of this fee if they show financial difficulty and furnish evidence of a previous year's payment of the fee.
Submit and Confirm: After finalizing the enrollment and listing, submit your information and confirm receipt from the FDA. Keep a record of your enrollment number for future reference. Notably, the FDA is required to respond to complete 510(k) submissions within 60 days, making timely submission crucial to avoid delays. Numerous hold-ups in the approval process can be prevented with adequate support, highlighting the significance of seeking expert compliance assistance.
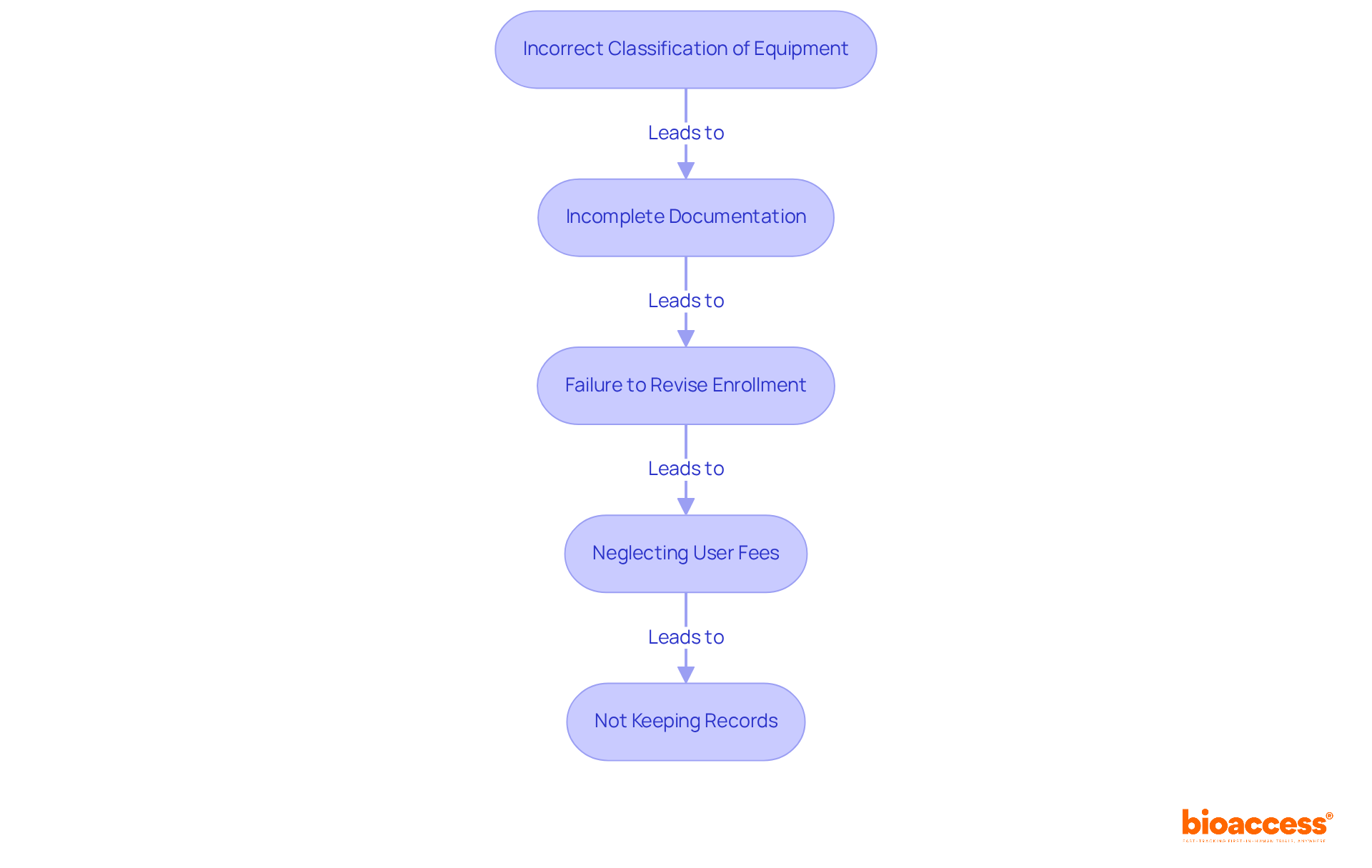
To ensure a successful registration process, it is crucial to be mindful of these common mistakes:
Incorrect Classification of Equipment: Misclassifying your equipment can lead to inappropriate regulatory pathways, resulting in significant delays and increased costs. Accurate classification is essential; manufacturers should assess the intended use, risk profile, and technological characteristics of their devices to avoid misclassification pitfalls.
Incomplete Documentation: Ensure that all necessary information is provided during sign-up and listing. Incomplete submissions are a primary reason for rejections in the FDA device listing and registration process, which can postpone market entry and raise development expenses. Consistency in documentation, particularly in the intended use statement, is crucial for FDA device listing and registration to prevent confusion and potential rejection.
Failure to Revise Enrollment: Remember to revise your enrollment each year and whenever there are alterations to your manufacturing processes or equipment information. Ignoring this can result in compliance issues that could endanger your device's market position concerning FDA device listing and registration.
Neglecting User Fees: Failing to pay the required user fees on time can lead to penalties or loss of registration status. Be proactive in managing these fees to maintain compliance and avoid disruptions.
Not Keeping Records: Maintain thorough records of all submissions and communications with the FDA. This documentation is crucial for future reference and compliance audits, especially regarding FDA device listing and registration, ensuring that you can demonstrate adherence to regulatory requirements effectively.
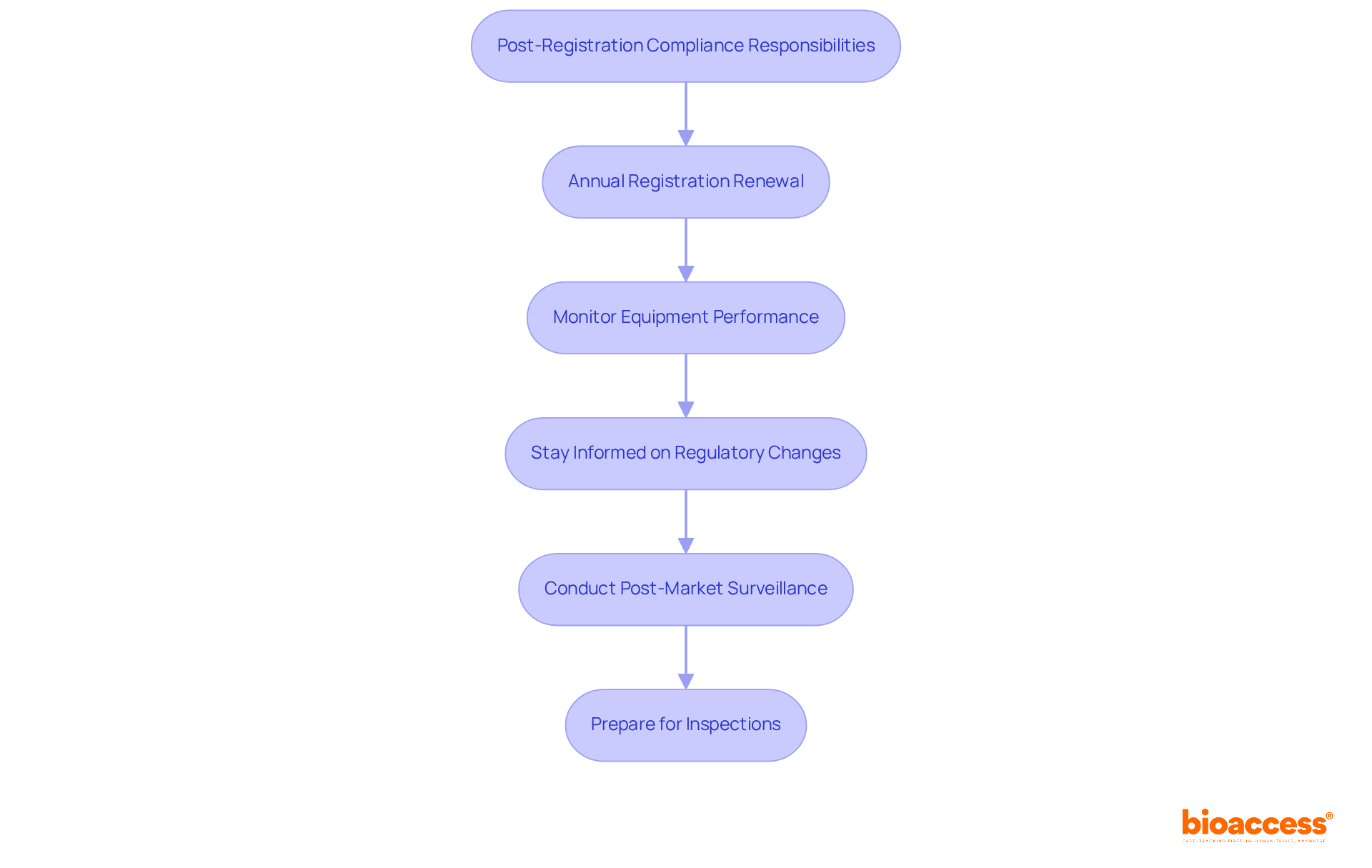
Maintaining compliance with the FDA device listing and registration after your product is registered is essential for ensuring safety and efficacy. The following key responsibilities are critical to manage:
Annual Registration Renewal: It is mandatory to review and renew your registration annually between October 1 and December 31, regardless of whether any changes have occurred. This process is crucial for maintaining compliance with the FDA device listing and registration and avoiding penalties.
Monitor Equipment Performance: Continuously assess your equipment's performance in the market. Promptly report any adverse events, serious injuries, or product defects to the FDA, as manufacturers must submit a Medical Device Report (MDR) within 30 calendar days of awareness for reportable incidents.
Stay Informed on Regulatory Changes: Regularly update yourself on any changes in FDA regulations that could affect your FDA device listing and registration or manufacturing processes. This proactive approach helps mitigate risks associated with non-compliance.
Conduct Post-Market Surveillance: Implement a robust post-market surveillance plan to gather data on your product's performance and safety in real-world settings. This is vital for identifying potential issues early and ensuring ongoing product safety.
Prepare for Inspections: Be ready for FDA inspections by maintaining organized records and ensuring compliance with all applicable regulations. Conducting regular internal audits can help identify and address potential issues before an official inspection occurs, thereby enhancing your compliance posture.
As the medical device industry is projected to reach $678.88 billion by 2025, expanding at a CAGR of 6%, maintaining compliance is essential for staying competitive in this growing market. With experts like Ana Criado and Katherine Ruiz, who have extensive experience in regulatory affairs and biomedical engineering, organizations can navigate these responsibilities effectively.
Navigating the FDA device listing and registration process is an essential step for any medical device manufacturer aiming for market entry in the United States. Understanding the intricacies of this regulatory framework not only ensures compliance but also significantly enhances the chances of successful product approval and ongoing market presence.
This article outlines critical steps, from determining equipment classification to submitting necessary documentation and managing post-registration responsibilities. Key insights include:
Recognizing common pitfalls—such as incorrect classification and incomplete submissions—can save manufacturers from costly delays and compliance issues.
In a rapidly evolving medical device landscape, staying informed and prepared is paramount. As the industry continues to expand, the significance of meticulous adherence to FDA regulations cannot be overstated. Manufacturers are encouraged to leverage expert guidance and maintain a proactive approach to compliance, ensuring their products not only meet regulatory standards but also contribute to the safety and efficacy of medical care.
What is the purpose of FDA device registration and listing?
The FDA device registration and listing process informs the FDA of the manufacturing establishment's location and specifies the particular items produced, which is crucial for maintaining a comprehensive overview of all medical devices on the market for safety and efficacy monitoring.
Who is Ana Criado and what is her expertise related to FDA device registration?
Ana Criado is the Director of Compliance Affairs with extensive expertise in FDA device registration. She has held leadership roles at Colombia’s oversight agency, INVIMA, and has experience as a biomedical engineering educator and external regulatory advisor for international firms.
What is the annual fee for FDA Device Facility enrollment in fiscal year 2025?
The annual fee for FDA Device Facility enrollment in fiscal year 2025 is set at $9,280.
When must establishments update their enrollment and listing with the FDA?
Establishments must update their enrollment and listing annually between October 1 and December 31 to ensure that the FDA's records remain current and precise.
What percentage of 510(k) applications received a Substantially Equivalent decision in the year ending September 2022?
Approximately 85 percent of 510(k) applications received a Substantially Equivalent decision, indicating regulatory approval for market sale in the U.S.
What happens to the 15 percent of submissions that did not achieve a Substantially Equivalent status?
The 15 percent of submissions that did not achieve Substantially Equivalent status highlight the importance of adhering to listing requirements to avoid potential market access issues.
What is a unique FDA Establishment Identifier (FEI) and why is it important?
A unique FDA Establishment Identifier (FEI) is assigned during enrollment and is vital for effectively tracking and managing compliance with FDA regulations.
Why is understanding the FDA device listing and registration process important?
Understanding the FDA device listing and registration process is the initial step toward ensuring compliance with FDA regulations, which facilitates smoother market entry for medical products.