


The article underscores the critical importance of mastering FDA product codes in the realm of medical device development, particularly emphasizing their pivotal role in regulatory submissions and classification.
It asserts that a comprehensive understanding of these codes is essential for Medtech innovators aiming to navigate complex regulatory requirements effectively.
This knowledge not only streamlines the approval process but also enhances the likelihood of successful market entry.
Proper classification is fundamental, as it determines the necessary regulatory pathway for devices, thereby shaping the overall development strategy.
Understanding the intricacies of FDA product codes is essential for anyone involved in the medical device industry. These codes classify devices based on their intended use and technological features, playing a crucial role in the regulatory approval process. As Medtech innovators strive to bring their products to market, they confront the challenge of navigating complex regulations that can significantly impact their success.
How can manufacturers effectively leverage FDA product codes to streamline their development processes and enhance their chances of approval?
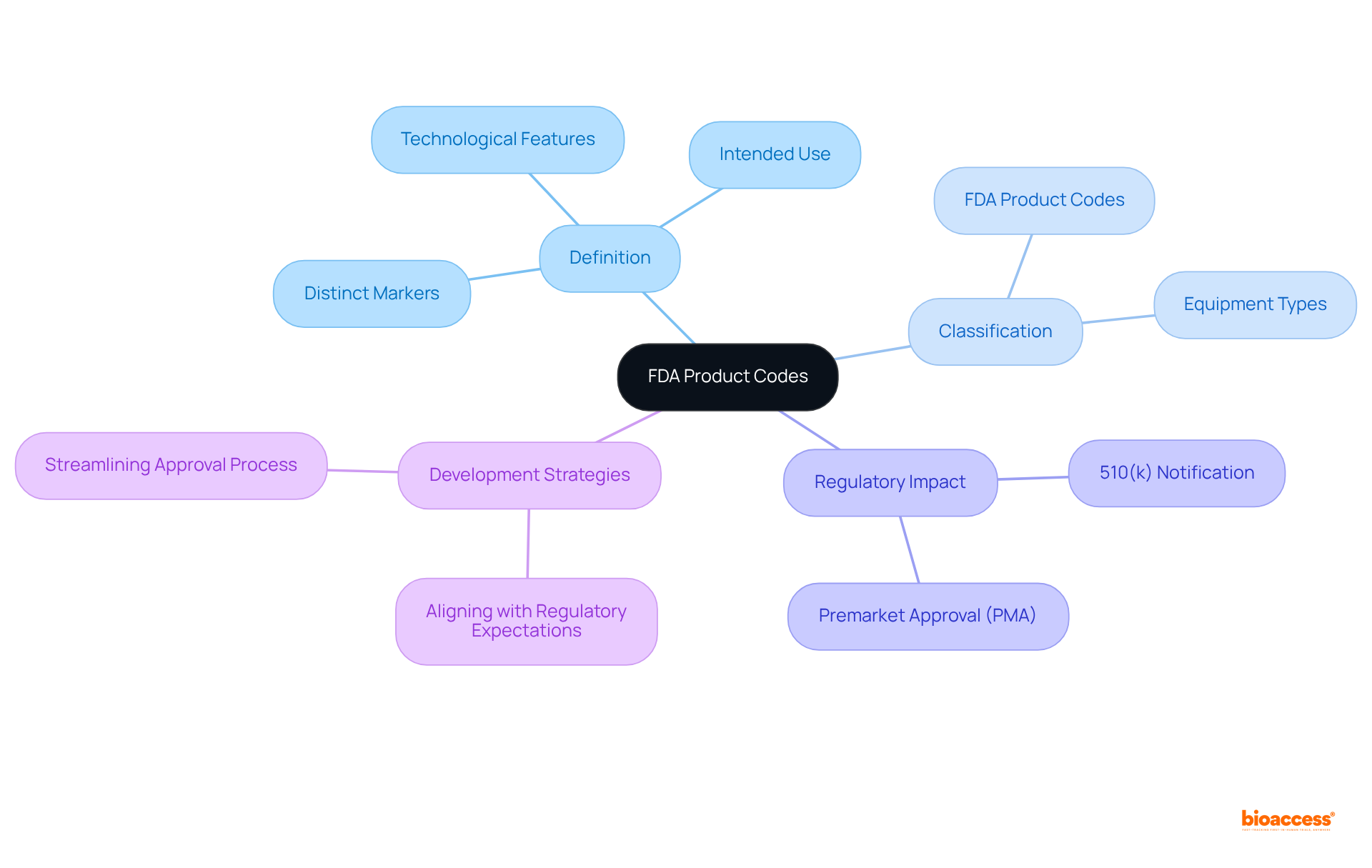
FDA identifiers serve as distinct markers allocated to medical instruments, classifying them according to their intended use and technological features. Each product identifier corresponds to a specific equipment type and the associated FDA product codes are crucial for regulatory submissions. Understanding these standards is essential for Medtech innovators as they navigate the complex landscape of FDA regulations. Proper classification can significantly streamline the approval process, ensuring that products reach the market efficiently and safely. For instance, FDA product codes can determine whether an item necessitates a 510(k) premarket notification or a more rigorous Premarket Approval (PMA). This comprehension enables manufacturers to align their development strategies with regulatory expectations, ultimately enhancing their chances of successful market entry.
For more information, you can refer to the FDA Product Code Classification Database.
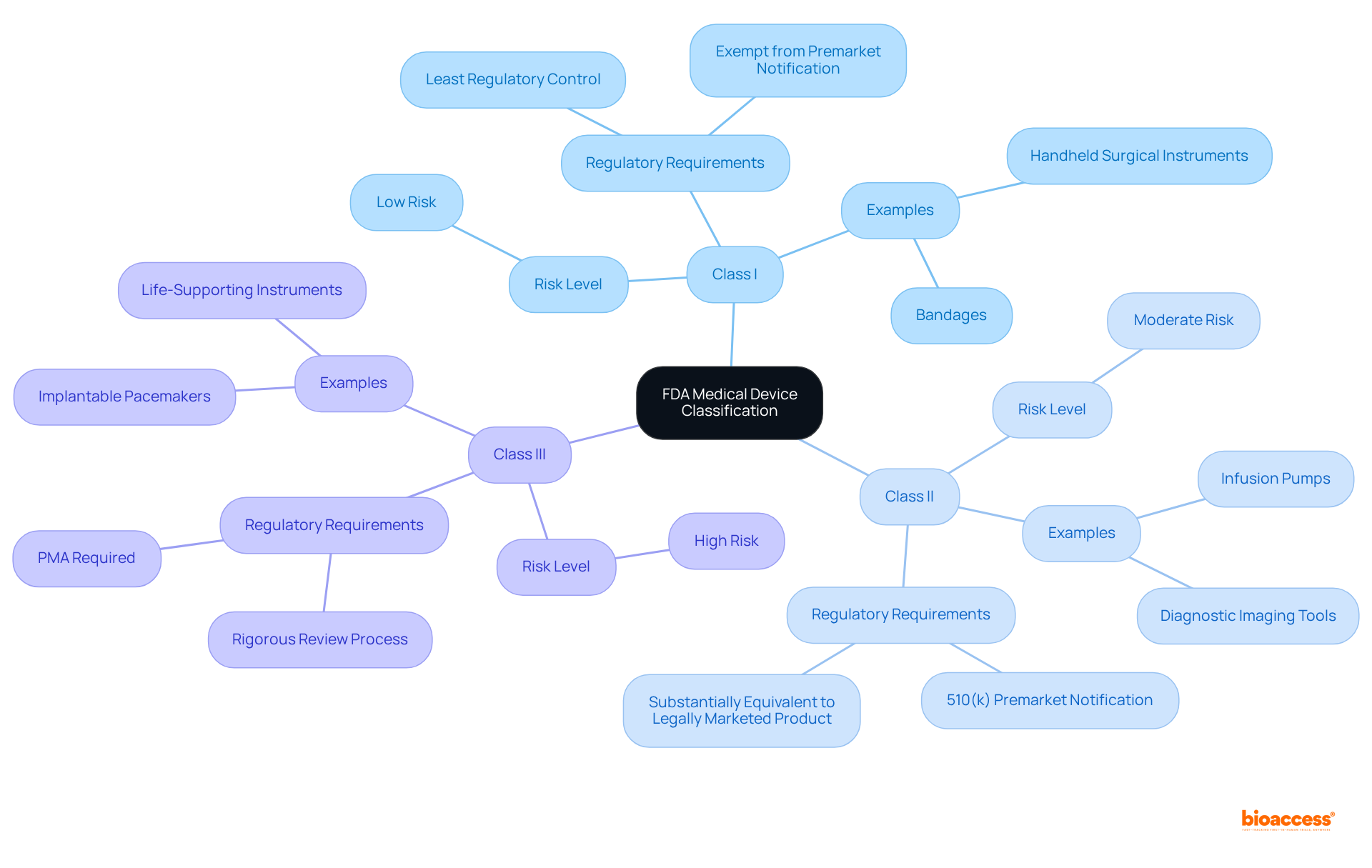
The FDA classifies medical devices into three categories based on their risk to patients and the level of regulatory control necessary:
Class I: These items are regarded as low risk and are subject to the least regulatory control. Examples include bandages and handheld surgical instruments. Most Class I products are exempt from premarket notification.
Class II: These products pose moderate risk and typically require a 510(k) premarket notification to demonstrate that they are substantially equivalent to a legally marketed product. Examples include infusion pumps and diagnostic imaging tools.
Class III: These items are high-risk and usually require a PMA, which involves a more rigorous review process. Examples include implantable pacemakers and specific life-supporting instruments.
Understanding these classifications, particularly the FDA product codes, is crucial for Medtech companies, as it directly influences their regulatory strategy and the resources required for compliance. For further details, visit the FDA Overview of Medical Device Classification.
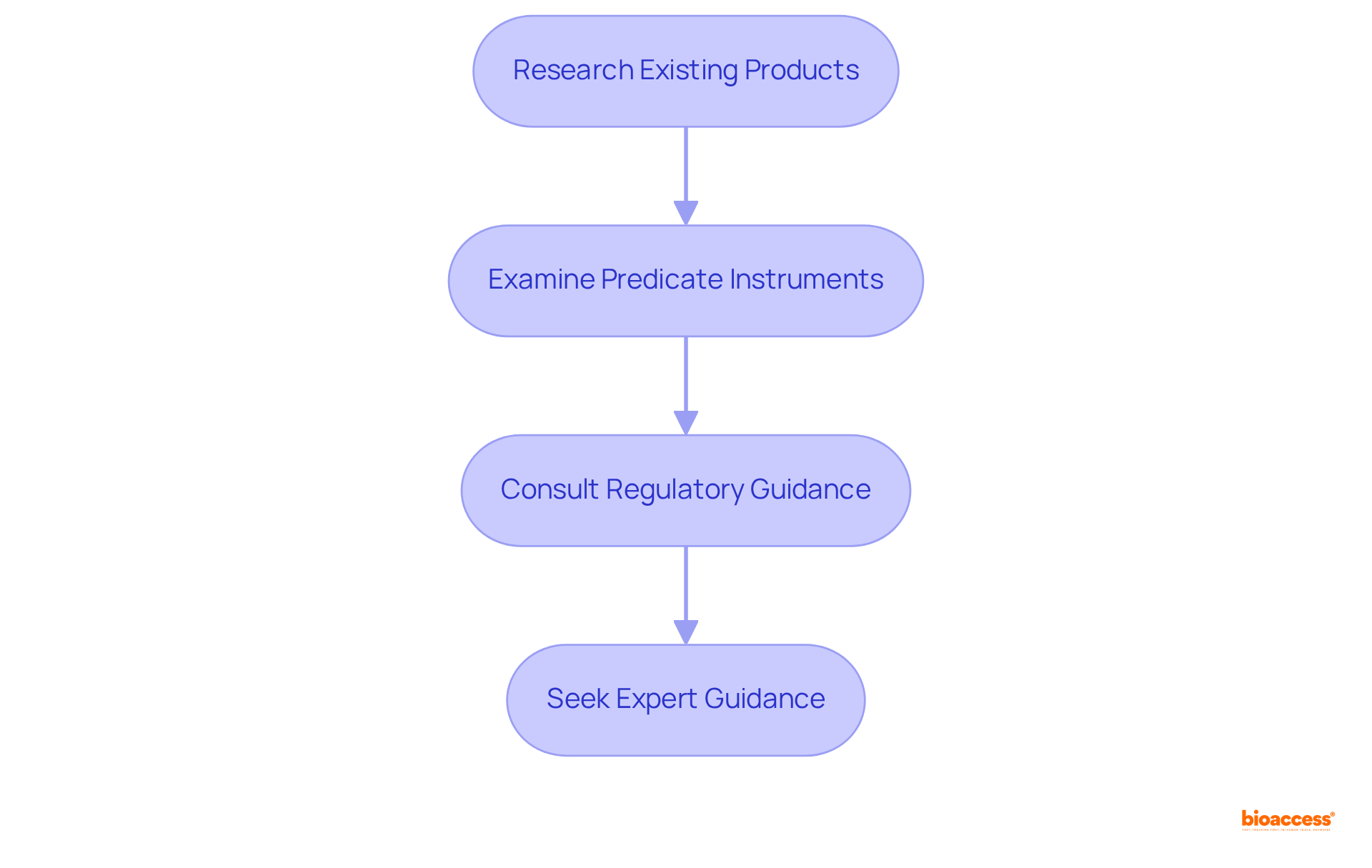
To identify the applicable FDA product code for your medical device, follow these essential steps:
Research Existing Products: Utilize the FDA product codes to search for items similar to yours in the FDA Product Code Classification Database. Focus on products with comparable intended uses and technological features to establish a relevant benchmark.
Examine Predicate Instruments: Review the identifiers assigned to predicate instruments—those that have already received FDA product codes—to comprehend the classification criteria that govern your device.
Consult Regulatory Guidance: Refer to FDA guidance documents that outline the classification process and provide insights into specific classifications and FDA product codes pertinent to your device type, ensuring you are well-informed.
Seek Expert Guidance: If necessary, consult regulatory affairs specialists or legal professionals who focus on FDA submissions. Their expertise can help you select the appropriate item identifier with confidence.
By precisely recognizing the item identifier, you can streamline your regulatory submissions and enhance your device's likelihood of approval.
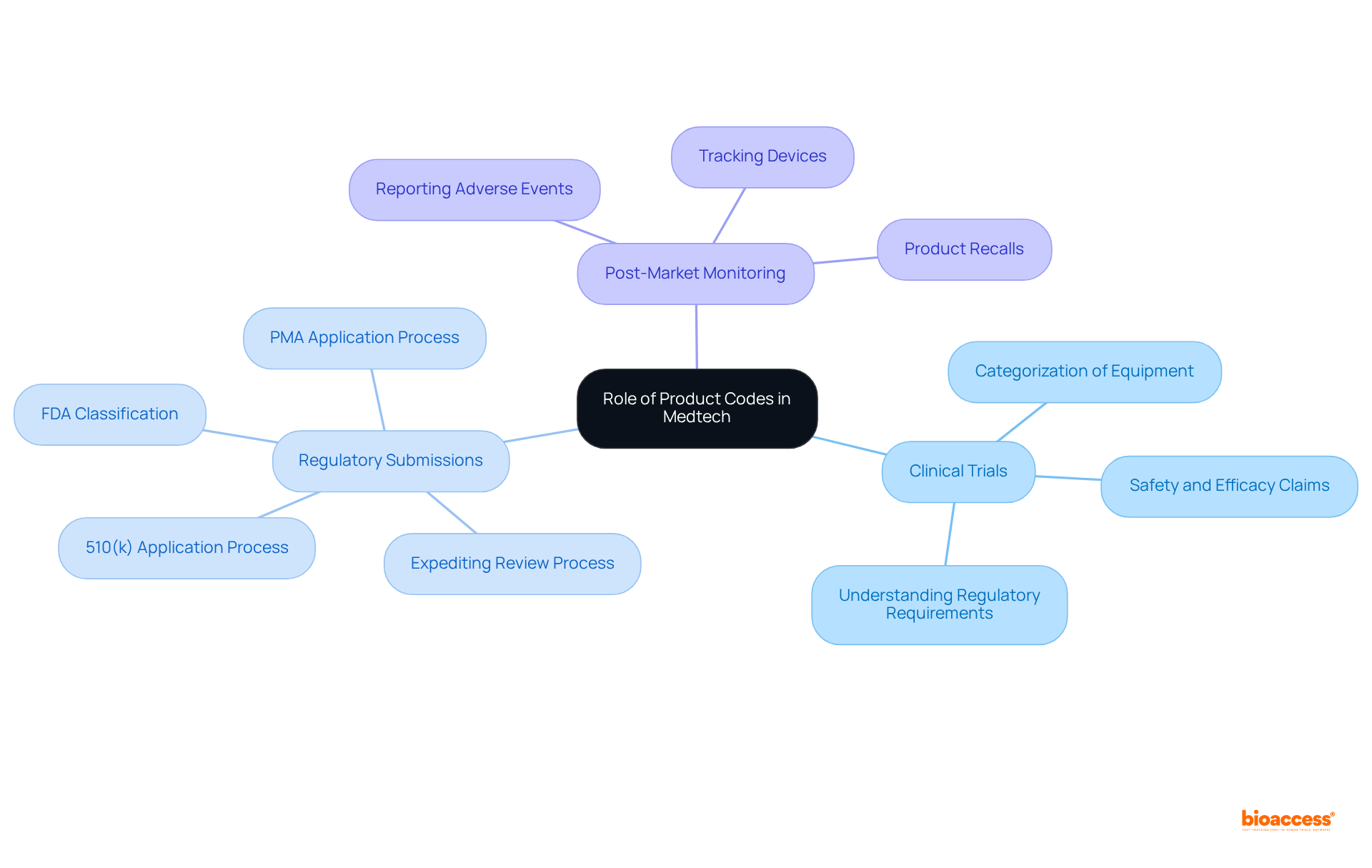
Product identifiers play a pivotal role in both clinical trials and regulatory submissions, underscoring their significance in the Medtech landscape. Here’s how:
Clinical Trials: In the planning stages of clinical trials, understanding the item identifier is essential for identifying the regulatory requirements pertinent to the study. The categorization of equipment directly influences the type of information necessary to substantiate safety and efficacy claims.
Regulatory Submissions: During the submission process, the item identifier serves as a key component of the 510(k) or PMA application. It aids the FDA in classifying the apparatus and evaluating the appropriate regulatory pathway. Accurate product identification can expedite the review process and reduce the likelihood of delays.
Post-Market Monitoring: Additionally, product identifiers are crucial for post-market monitoring activities, including the reporting of adverse events and product recalls. They ensure that the FDA can effectively track devices, thereby maintaining public safety.
In summary, a thorough understanding of product codes is vital for Medtech companies aiming to navigate the regulatory landscape successfully. For further insights, refer to the FDA Guidance on Medical Device Classification.
Mastering FDA product codes is essential for effective medical device development, as these identifiers serve as the backbone of regulatory compliance. Understanding how to navigate the classification system not only aids in aligning product development with regulatory expectations but also enhances the likelihood of successful market entry.
Key insights discussed include:
In conclusion, a comprehensive grasp of FDA product codes and their implications is vital for anyone involved in the Medtech industry. As the landscape of medical device development continues to evolve, staying informed about regulatory requirements and product classifications empowers manufacturers to navigate challenges effectively and ensure the safety and efficacy of their devices. Taking proactive steps in understanding these codes can ultimately lead to more efficient approval processes and better healthcare outcomes.
What are FDA product codes?
FDA product codes are distinct markers allocated to medical instruments that classify them based on their intended use and technological features.
Why are FDA product codes important in Medtech?
FDA product codes are crucial for regulatory submissions, as they help streamline the approval process and ensure that products reach the market efficiently and safely.
How do FDA product codes impact the regulatory process?
FDA product codes can determine whether a medical device requires a 510(k) premarket notification or a more rigorous Premarket Approval (PMA), influencing the development strategies of manufacturers.
How can understanding FDA product codes benefit Medtech innovators?
Understanding FDA product codes allows Medtech innovators to align their development strategies with regulatory expectations, enhancing their chances of successful market entry.
Where can I find more information about FDA product codes?
More information can be found in the FDA Product Code Classification Database.