


This article examines the types, production methods, and applications of fragment antibodies, underscoring their critical role in therapeutic and diagnostic contexts. Fragment antibodies, including Fab, scFv, and VHH, are specifically engineered to enhance tissue penetration and specificity. Their significance is particularly pronounced in cancer treatment and disease diagnostics. The projected market growth for these antibodies reflects their escalating importance in the realm of personalized medicine, highlighting the necessity for ongoing research and collaboration in this dynamic field.
Fragment antibodies are revolutionizing the landscape of medical research and treatment, offering a unique blend of specificity and versatility that full-sized antibodies often lack. These engineered proteins, including types such as Fab, scFv, and VHH, are designed to maintain their antigen-binding capabilities while enhancing tissue penetration and reducing immunogenicity. As the demand for targeted therapies grows, the challenge remains: how can researchers and clinicians harness the full potential of fragment antibodies in both diagnostics and therapeutics? This article delves into the intricacies of fragment antibody techniques, exploring their types, production methods, and diverse applications that are shaping the future of personalized medicine.
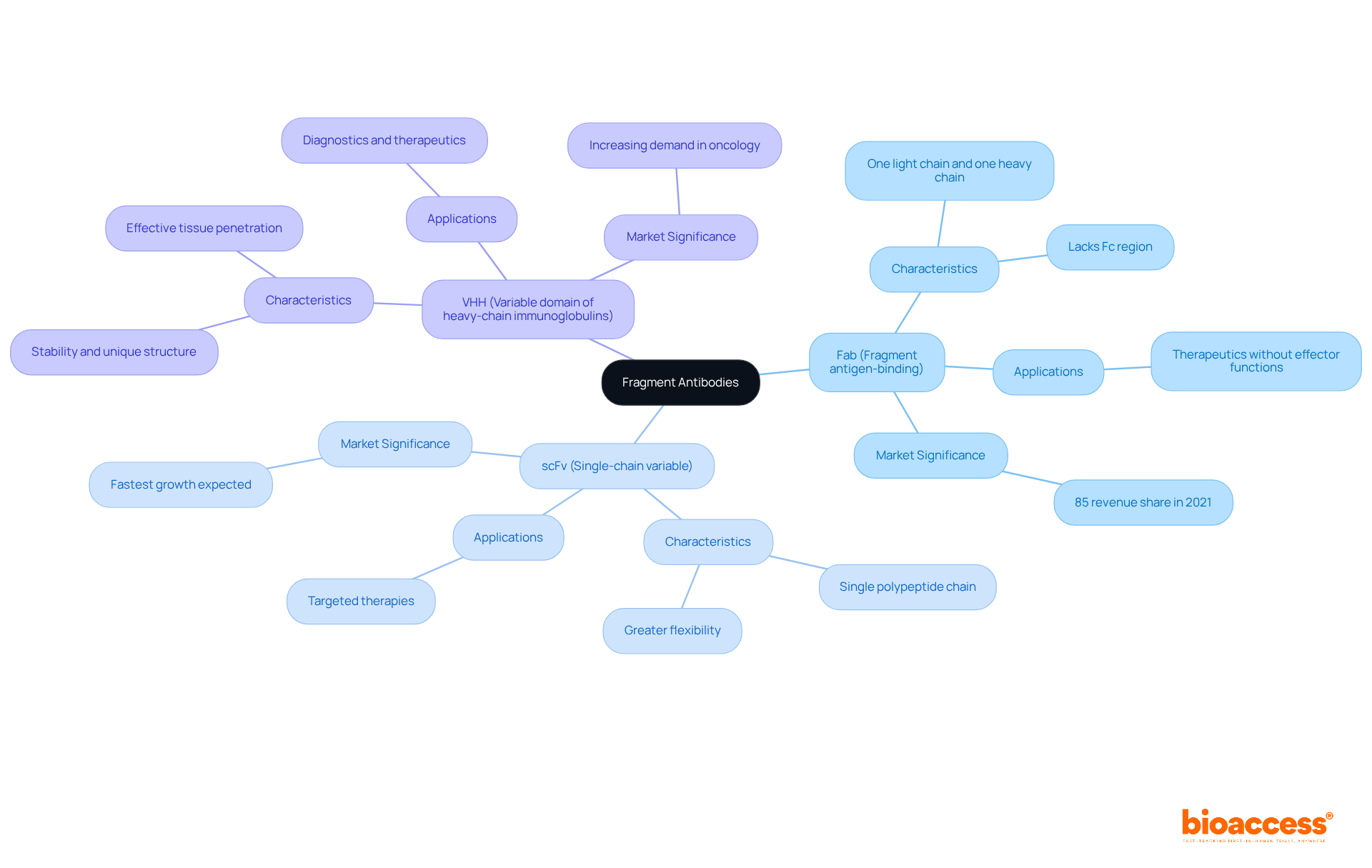
Fragment proteins are engineered, smaller versions of full-sized proteins, designed to retain essential antigen-binding capabilities while offering enhanced properties such as improved tissue penetration and reduced immunogenicity. The primary types of fragment antibodies include:
Fab (Fragment antigen-binding): Comprising one light chain and one heavy chain, Fab fragments maintain the ability to bind to antigens but lack the Fc region, which is responsible for effector functions. This trait renders them especially beneficial in treatment scenarios where effector functions are not necessary.
scFv (Single-chain variable): This type consists of a single polypeptide chain that combines the variable regions of both heavy and light chains, resulting in a smaller size and greater flexibility in applications. The compact nature of scFvs enables easier handling and integration into various treatment modalities, including targeted therapies.
VHH (Variable domain of heavy-chain immunoglobulins): Originating from camelids, VHH components are distinguished by their stability and capacity to attach to targets that are frequently unreachable by traditional immunoglobulins. Their distinctive structure allows them to infiltrate tissues more effectively, making them valuable in both diagnostic and treatment contexts.
These proteins, particularly fragment antibodies, serve a crucial function in numerous uses, such as diagnostics and therapeutics, due to their reduced size and improved capacity to infiltrate tissues relative to full-length proteins. The increasing need for specialized treatments, particularly in cancer care and immune system studies, underscores the significance of small protein components in medical research and clinical applications. Notably, the immunodeficiency segment held over 85.0% revenue share in 2021, highlighting the significant market demand for these therapies. As the market for immune protein pieces is expected to attain USD 11.71 billion by 2030, increasing from USD 7.19 billion in 2022, the importance of these components in enhancing personalized medicine and boosting treatment effectiveness continues to grow. Furthermore, the FDA and EMA's expedited routes for novel biologics are anticipated to facilitate the creation and authorization of segmented proteins, improving their accessibility in the marketplace. The Asia Pacific region is also expected to experience significant growth in this market, driven by advancements in biotechnology and increasing healthcare needs.
Fragment antibodies can be classified according to their structure and function, emphasizing their importance in clinical research and therapeutic applications.
Fab Fragments: Generated through enzymatic cleavage of full-length antibodies, Fab fragments consist of two antigen-binding sites per molecule. Their strong binding abilities make fragment antibodies essential in medical applications, particularly in addressing autoimmune disorders and cancers. Notably, three FDA-approved Fab therapeutics include abciximab, ranibizumab, and certolizumab pegol, showcasing their clinical efficacy. Ongoing trials, such as those comparing ranibizumab with bevacizumab for age-related macular degeneration, further emphasize their significance in the market.
scFv Components: Single-chain variable components (scFvs) are engineered by linking the variable regions of heavy and light chains with a flexible peptide linker. Their smaller size enhances tissue penetration, making them ideal for imaging and targeted therapies. ScFvs represent roughly 40% of clinically assessed components, underscoring their increasing importance in precision medicine.
VHH Units: Also referred to as nanobodies, VHH units are derived from heavy-chain immunoglobulins found in camelids. Their unique structure enables effective targeting of hidden epitopes, and they can be produced in microbial systems, which reduces production costs. VHHs are gaining traction in treatment applications due to their stability and high specificity.
F(ab')2 Portions: Created through the pepsin digestion of IgG immunoglobulins, F(ab')2 portions consist of two Fab components connected by a disulfide bond. This configuration offers improved stability and binding affinity, making them suitable for various medical and diagnostic uses.
Each type of molecule, such as fragment antibodies, possesses unique characteristics that serve specific purposes, including diagnostics, therapeutics, and research. However, challenges such as the rapid deterioration of fragments lacking Fc domains must be considered, as this impacts their functionality and stability. The ongoing advancements in immune molecule engineering continue to expand the potential applications of these components, particularly in oncology and immunotherapy. As specialists highlight, the modular nature of immune proteins facilitates innovative therapeutic designs that may lead to new component types undergoing clinical assessment.

The production of fragment antibodies employs various techniques, each offering distinct advantages:
Enzymatic Digestion: This conventional method employs proteolytic enzymes such as papain or pepsin to cut full-length immunoglobulins into smaller pieces. While simple, it requires careful optimization to attain the desired specificity and yield.
Recombinant DNA Technology: Utilizing genetic engineering, this approach generates particular protein segments in host cells like bacteria or yeast. Techniques such as phage display aid in the selection of high-affinity pieces from extensive libraries, significantly improving production efficiency. Recent studies have demonstrated the effectiveness of this approach, with artificial neural networks (ANN) predicting protein yields that closely match experimental results, showcasing its reliability in optimizing production conditions with fragment antibody. For instance, optimal culture conditions for protein yield were identified as induction at a cell density of 0.7 and isopropyl β-D-1-thiogalactopyranoside concentration of 0.6 mM for 32 hours at 30°C, yielding 259.51 mg/L of protein.
Cell-Free Protein Synthesis: This groundbreaking method enables the quick and scalable creation of immune protein sections without depending on living cells. By utilizing cell-free systems, researchers can streamline the production process, reducing time and costs associated with traditional methods.
Transgenic Animals: Fragment proteins can also be generated in transgenic animals, which express the desired proteins in their milk or serum. This biological source provides a viable option for purification, although it involves longer development times and higher costs.
Recent FDA approvals, including Brolucizumab for age-related macular degeneration and Tebentafusp for melanoma, emphasize the clinical significance of small protein agents. Each method presents unique challenges and benefits, with the choice of technique often influenced by the intended application and required yield of the fragment antibody. The continuous progress in recombinant DNA technology keeps improving the efficiency and effectiveness of molecule production, leading to innovative treatment solutions. As noted by expert Sama Pirkalkhoran, using smaller-sized fragments can improve potency by increasing the effective dose with a higher density of target binding in a given volume.

Fragment antibodies offer a diverse array of applications in both therapeutic and diagnostic contexts.
Therapeutic Uses: Fragment proteins are increasingly pivotal in cancer treatment, owing to their smaller size that facilitates superior tumor penetration and minimizes off-target effects. These immune proteins can be meticulously designed to specifically target cancer antigens, significantly enhancing treatment effectiveness. The market for these therapeutic applications is anticipated to grow at a compound annual growth rate (CAGR) of 5.9% from 2022 to 2030, propelled by the rising incidence of cancer, with an estimated 1.9 million new cases and approximately 609,360 cancer deaths projected in the U.S. in 2022 alone.
Diagnostic Applications: In the realm of diagnostics, small proteins are indispensable for tests such as ELISAs and immunohistochemistry. Their high specificity for antigens renders them invaluable for early disease detection, thereby contributing to improved patient outcomes. The market for immune proteins was evaluated at around USD 7.19 billion in 2022, underscoring the escalating demand for diagnostic applications.
Imaging: Fragment proteins are also employed in molecular imaging methods, providing high-resolution images of tumors and other pathological conditions. Their rapid clearance from the bloodstream enhances imaging clarity, making them essential for precise diagnostics.
In research settings, fragment antibodies are critical tools for investigating protein interactions, cellular activities, and disease mechanisms. Their versatility enables a broad spectrum of experimental applications, further propelling advancements in medical research.
In summary, the unique properties of fragment antibodies position them as vital components in advancing medical research and enhancing patient outcomes across a multitude of therapeutic and diagnostic landscapes.

Fragment antibodies signify a remarkable progression in immunotherapy and diagnostics, equipping researchers and clinicians with versatile tools that enhance treatment efficacy and diagnostic precision. By concentrating on smaller, engineered versions of antibodies, such as Fab, scFv, and VHH, these fragments present unique characteristics that improve tissue penetration and diminish immunogenicity, rendering them invaluable in contemporary medicine.
Throughout this discourse, critical insights into the types, production methods, and applications of fragment antibodies have been thoroughly examined. The diverse structures, ranging from Fab fragments to nanobodies, illustrate their specific roles in targeting antigens and facilitating therapeutic interventions. The production techniques, encompassing enzymatic digestion and recombinant DNA technology, underscore the innovative approaches employed to efficiently create these essential proteins. Furthermore, the therapeutic and diagnostic applications highlight their increasing significance in tackling complex medical challenges, particularly in oncology and disease detection.
The implications of fragment antibodies extend beyond their immediate applications; they epitomize a transition towards more personalized and effective treatment strategies. As the market for these innovative therapies continues to burgeon, propelled by escalating healthcare needs and advancements in biotechnology, the potential for fragment antibodies to transform patient care is substantial. Engaging with this evolving landscape not only fosters advancements in medical research but also paves the way for enhanced patient outcomes across various therapeutic contexts.
What are fragment antibodies?
Fragment antibodies are engineered, smaller versions of full-sized proteins designed to retain essential antigen-binding capabilities while offering enhanced properties such as improved tissue penetration and reduced immunogenicity.
What are the primary types of fragment antibodies?
The primary types of fragment antibodies include Fab (Fragment antigen-binding), scFv (Single-chain variable), and VHH (Variable domain of heavy-chain immunoglobulins).
What is a Fab fragment?
A Fab fragment comprises one light chain and one heavy chain, maintaining the ability to bind to antigens but lacking the Fc region responsible for effector functions, making them beneficial in scenarios where such functions are unnecessary.
What is an scFv fragment?
An scFv fragment consists of a single polypeptide chain that combines the variable regions of both heavy and light chains, resulting in a smaller size and greater flexibility for various applications, including targeted therapies.
What is a VHH fragment?
VHH fragments originate from camelids and are distinguished by their stability and ability to attach to targets that traditional immunoglobulins often cannot reach, allowing for effective tissue infiltration.
What roles do fragment antibodies play in medical applications?
Fragment antibodies are crucial in diagnostics and therapeutics due to their reduced size and improved capacity to infiltrate tissues, making them significant in cancer care and immune system studies.
What is the market outlook for fragment antibodies?
The market for immune protein pieces is projected to reach USD 11.71 billion by 2030, increasing from USD 7.19 billion in 2022, indicating a growing demand for these therapies, especially in personalized medicine.
How are regulatory bodies impacting the development of fragment antibodies?
The FDA and EMA's expedited routes for novel biologics are expected to facilitate the creation and authorization of segmented proteins, improving their accessibility in the marketplace.
Which region is expected to see significant growth in the fragment antibody market?
The Asia Pacific region is anticipated to experience significant growth in the fragment antibody market, driven by advancements in biotechnology and increasing healthcare needs.