


In the realm of clinical research, adherence to Good Clinical Practice (GCP) and ethical standards stands as a fundamental pillar, ensuring the safety and well-being of human participants. This is not merely a regulatory requirement; it is essential for the integrity of the research process. As the landscape of clinical trials evolves, mastering GCP and navigating ethics inspections by NAMMD becomes increasingly critical for research teams striving for success.
However, a pressing challenge arises: how can organizations effectively prepare for these inspections while fostering a culture of integrity and compliance? This article explores best practices that not only enhance readiness for GCP and ethics inspections but also contribute to the overall quality and efficacy of clinical research. By understanding these practices, research teams can better position themselves to meet regulatory expectations and improve their operational standards.
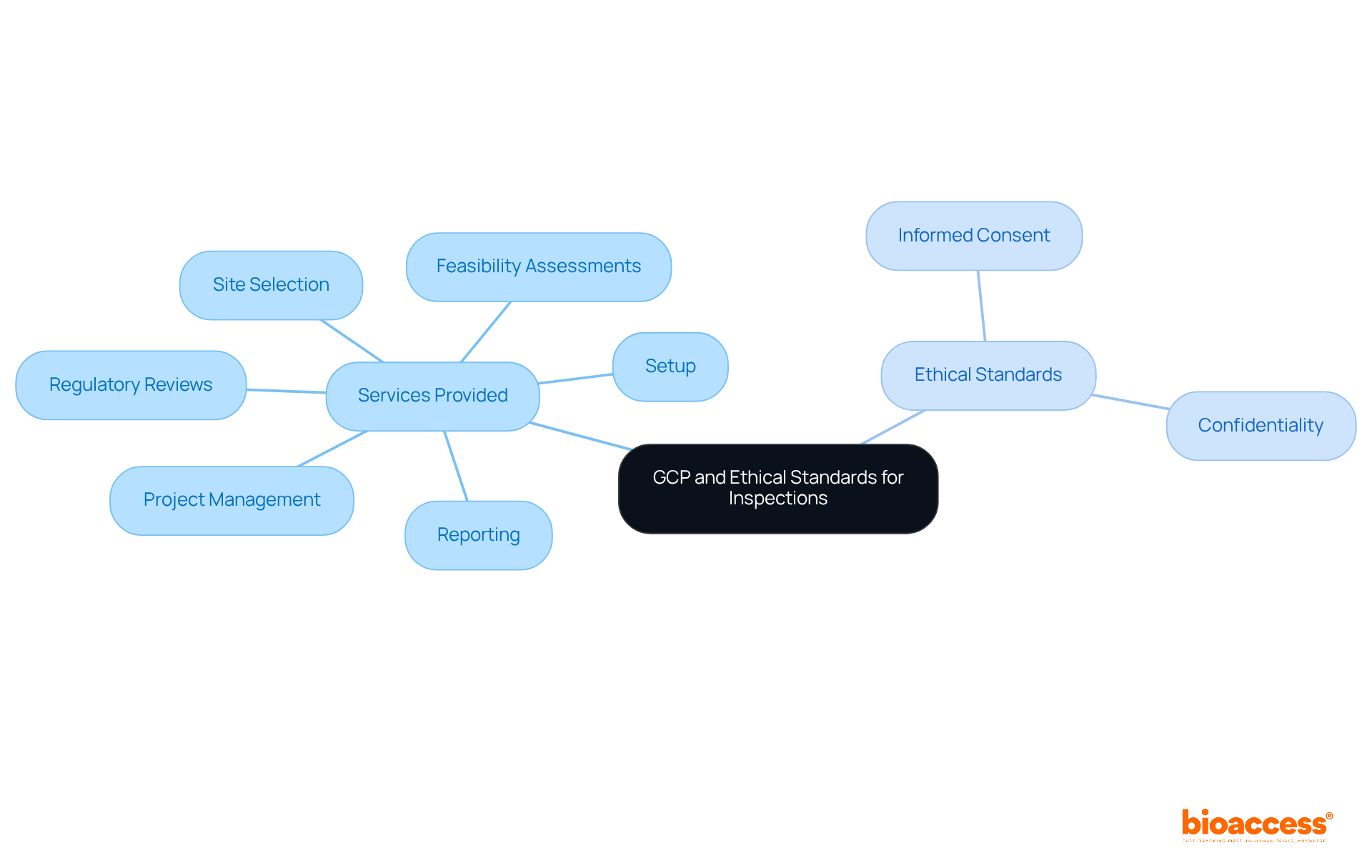
Good Clinical Practice (GCP) and ethics inspections by NAMMD represent an international ethical and scientific quality standard that is crucial for the design, conduct, recording, and reporting of studies involving human participants. Mastery of GCP is vital for ensuring that studies are conducted ethically, safeguarding the rights, safety, and well-being of participants. At bioaccess, we provide comprehensive research study management services, including:
During GCP and ethics inspections by NAMMD, ethical standards such as informed consent and confidentiality are of utmost importance. Adhering to these standards not only prepares research teams for GCP and ethics inspections by NAMMD but also fosters a culture of integrity within clinical research environments. Regular training and updates on GCP guidelines are essential, ensuring that all team members remain informed and compliant with evolving regulations. This proactive approach significantly enhances trial outcomes; studies show that well-trained teams are more likely to uphold ethical standards, ultimately minimizing the risk of trial failures and ensuring patient safety.
To ensure compliance with GCP and ethics inspections by nammd, it is essential to establish comprehensive documentation protocols and uphold ethical standards. This involves maintaining critical documents like study protocols, informed consent forms, and case report forms (CRFs). Each document must be meticulously filled out, leaving no blank fields or missing entries. By implementing a regulatory binder that organizes all essential documents, the inspection process can be streamlined effectively.
Regular audits of documentation practices are vital for identifying gaps and ensuring that all records remain accurate and up-to-date. Moreover, utilizing electronic data capture systems can significantly enhance data integrity and facilitate easier access during inspections. By prioritizing documentation, study groups can demonstrate their commitment to adherence and ethical conduct. As Anita Trupiano aptly states, "Inspection readiness should be approached as part of everyday practice rather than a last-minute response."
It's also crucial to be aware of common pitfalls, such as maintaining version control in informed consent procedures, to prevent potential issues during inspections. Bioaccess offers extensive research study management services, including:
These services are vital for ensuring adherence to GCP and ethics inspections by nammd.

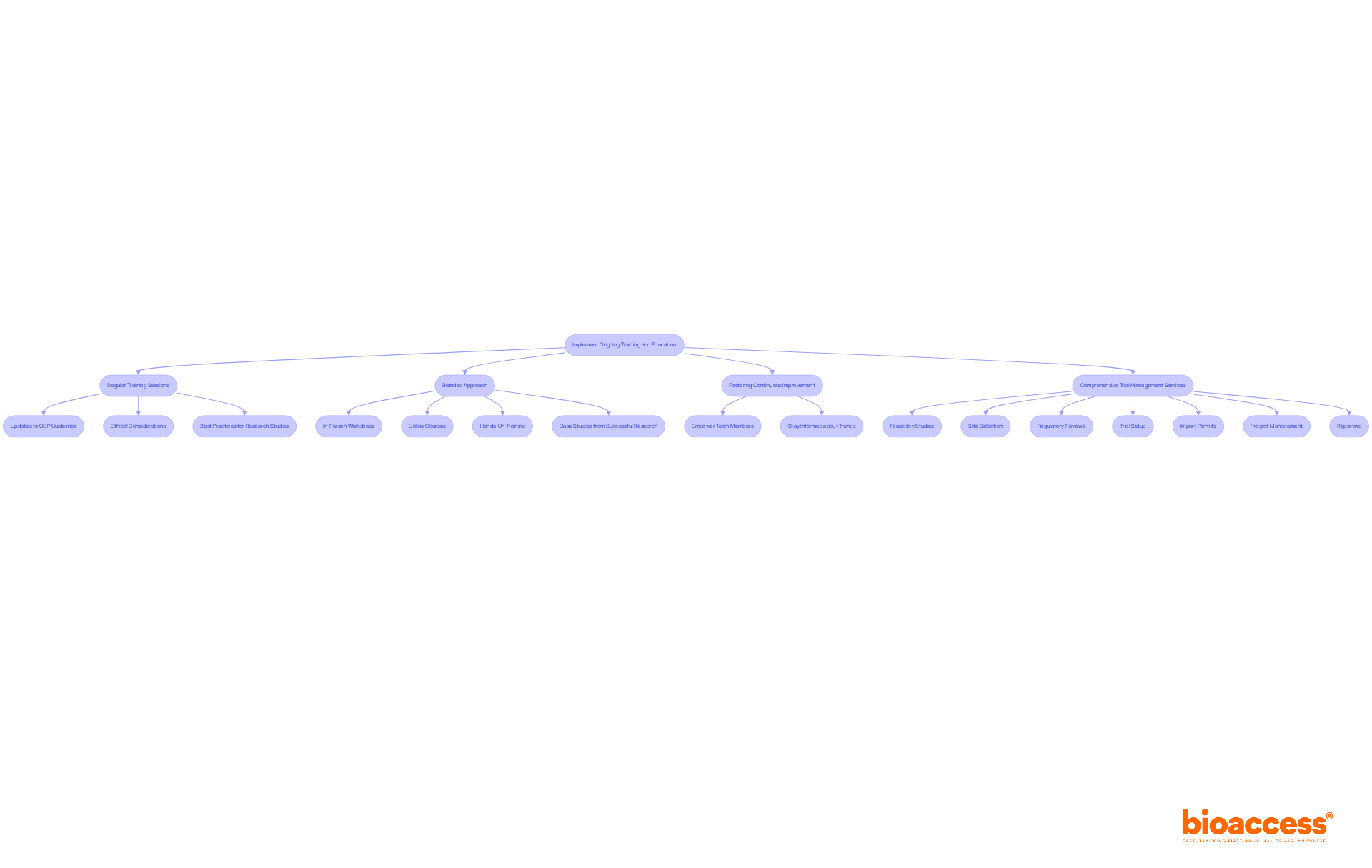
Ongoing training and education for research teams are essential for maintaining compliance with GCP and ethics inspections by NAMMD, as well as ethical standards. Regular training sessions must encompass updates to GCP guidelines, ethical considerations, and best practices for conducting research studies, as well as the importance of GCP and ethics inspections by NAMMD. A blended approach that includes in-person workshops, online courses, and hands-on training caters to diverse learning preferences, ensuring a comprehensive understanding of the material. For instance, incorporating case studies from successful research studies can effectively illustrate GCP training techniques in action.
Moreover, fostering a culture of continuous improvement empowers team members to stay informed about industry trends and regulatory changes. As Henry Ford wisely stated, "The only thing worse than training your employees and having them leave is not training them and having them stay." By prioritizing the training of investigation teams, organizations can significantly enhance operational efficiency and readiness for GCP and ethics inspections by NAMMD, ultimately driving success in healthcare.
Comprehensive trial management services - including feasibility studies, site selection, regulatory reviews, trial setup, import permits, project management, and reporting - are crucial components of this educational framework. These services not only bolster training initiatives but also positively influence local economies through job creation, economic growth, healthcare improvement, and international collaboration.
However, common pitfalls to avoid include:
By addressing these challenges, organizations can maximize the benefits of their training initiatives.
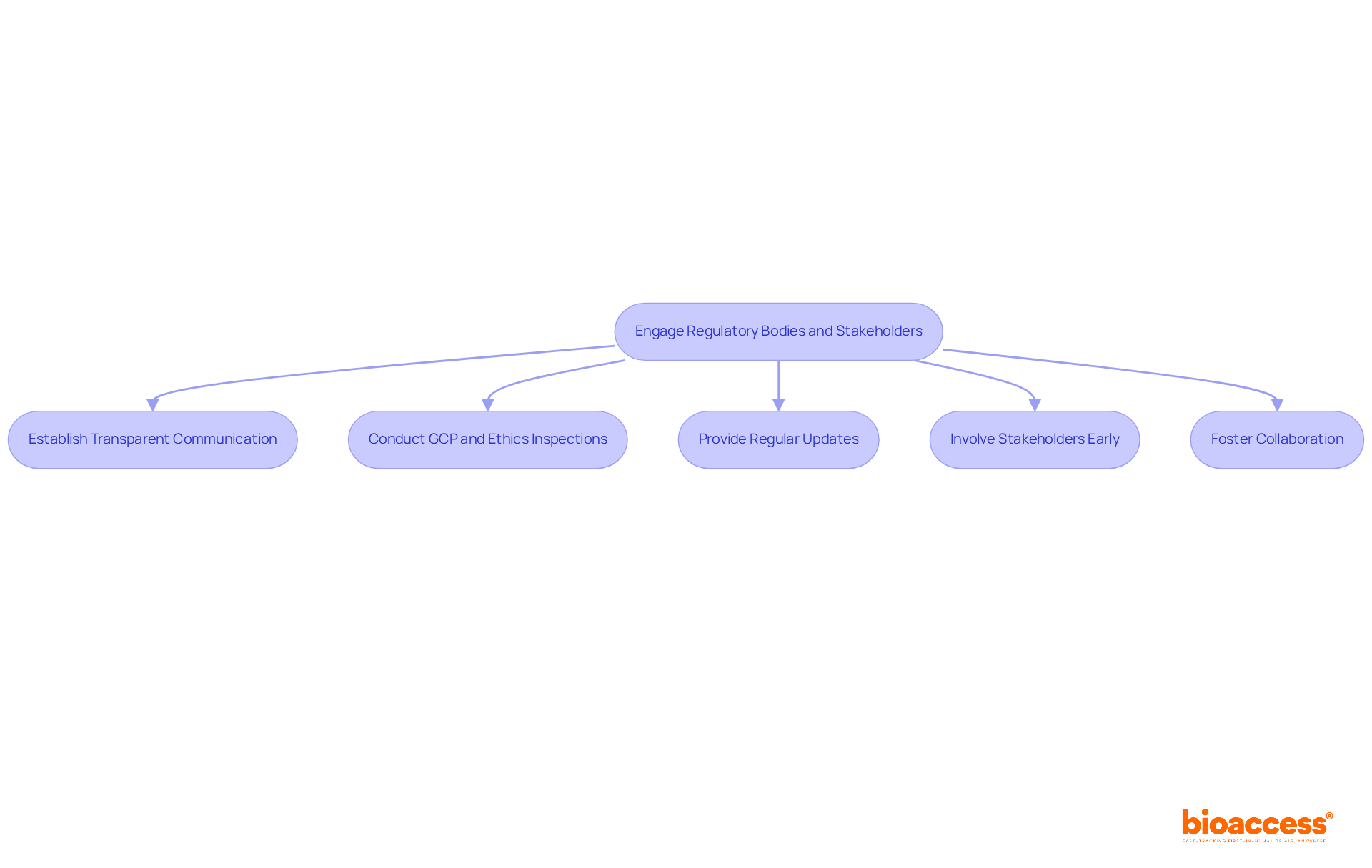
Effective interaction with regulatory bodies and stakeholders is essential for ensuring compliance and fostering collaboration in medical studies. By establishing transparent communication channels with the National Agency for Medicines and Medical Devices (NAMMD) and ensuring GCP and ethics inspections by NAMMD, expectations are clarified, and the inspection process is streamlined. Regular updates and meetings keep stakeholders informed about study progress and potential challenges, which enhances trust and cooperation.
Involving stakeholders early in the study design phase yields valuable insights that can significantly improve the feasibility and relevance of the research. The partnership between bioaccess™ and Caribbean Health Group aims to position Barranquilla as a premier location for medical studies in Latin America, supported by Colombia's Minister of Health during the gathering on March 29, 2019. This collaboration illustrates how strong connections with researchers, sponsors, and ethics boards facilitate smoother interactions during GCP and ethics inspections by NAMMD, which ultimately contributes to a more effective research process. By emphasizing stakeholder involvement, project teams cultivate a supportive atmosphere that not only encourages adherence but also enhances the overall quality and success of studies.
Furthermore, GlobalCare Clinical Trials' collaboration with bioaccess™ has led to an impressive over 50% decrease in recruitment time and 95% retention rates, underscoring the critical role of stakeholder involvement in research success. As we look toward 2025, collaboration with NAMMD will be pivotal in navigating the evolving landscape of clinical trials, especially concerning GCP and ethics inspections by NAMMD.
Mastering Good Clinical Practice (GCP) and adhering to the ethical standards established by NAMMD is crucial for achieving clinical success. A thorough understanding of these guidelines is not just essential for the ethical treatment of participants; it also significantly enhances the overall quality and reliability of clinical research outcomes.
Key insights highlight the importance of comprehensive documentation and compliance protocols. These measures ensure that every aspect of a study is meticulously recorded and readily accessible during inspections. Furthermore, ongoing training and education for research teams are vital, as a well-informed team is better equipped to navigate the complexities of GCP and ethics inspections. Engaging effectively with regulatory bodies and stakeholders further strengthens the research process, facilitating smoother interactions and fostering a culture of collaboration.
In summary, the pursuit of excellence in clinical trials relies heavily on a steadfast commitment to GCP and ethical practices. By prioritizing training, documentation, and stakeholder engagement, research teams can not only prepare for NAMMD inspections but also contribute to the advancement of medical research. Embracing these best practices will ultimately lead to improved patient safety, enhanced research integrity, and successful outcomes in the clinical landscape of 2025 and beyond.
What is Good Clinical Practice (GCP)?
Good Clinical Practice (GCP) is an international ethical and scientific quality standard that governs the design, conduct, recording, and reporting of studies involving human participants.
Why is mastery of GCP important?
Mastery of GCP is vital for ensuring that studies are conducted ethically, safeguarding the rights, safety, and well-being of participants involved in clinical research.
What services does bioaccess provide in relation to research study management?
Bioaccess provides comprehensive research study management services, including feasibility assessments, site selection, regulatory reviews, setup, project management, and reporting.
What ethical standards are emphasized during GCP and ethics inspections by NAMMD?
During GCP and ethics inspections by NAMMD, ethical standards such as informed consent and confidentiality are of utmost importance.
How do GCP and ethics inspections benefit research teams?
Adhering to GCP and ethical standards prepares research teams for inspections, fosters a culture of integrity, and enhances the overall quality of clinical research.
Why is regular training on GCP guidelines essential?
Regular training and updates on GCP guidelines are essential to ensure that all team members remain informed and compliant with evolving regulations, which significantly enhances trial outcomes.
How does well-trained research teams impact trial outcomes?
Studies show that well-trained teams are more likely to uphold ethical standards, ultimately minimizing the risk of trial failures and ensuring patient safety.