


The enforcement of Good Clinical Practice (GCP) by the National Agency for Medicines and Medical Devices (NAMMD) is essential for upholding the integrity and safety of clinical research involving human subjects. By adhering to internationally accepted ethical standards, researchers not only protect participants but also bolster the credibility of their findings. Yet, a pressing question arises: how can organizations ensure compliance in the face of evolving regulations and the complexities inherent in clinical trials? This article explores the fundamental principles of GCP, the enforcement mechanisms utilized by NAMMD, and the best practices for cultivating a culture of compliance that ultimately drives successful research outcomes.
The good clinical practice enforcement by nammd serves as a vital framework of internationally accepted ethical and scientific quality standards, essential for the design, conduct, recording, and reporting of studies involving human subjects. The core principles include:
At bioaccess®, we recognize that understanding and adhering to these principles is crucial for researchers and sponsors to ensure compliance with regulatory standards and uphold the integrity of research. Our comprehensive research study management services - including feasibility assessments, site selection, regulatory reviews, study setup, import permits, project management, and reporting - are designed to support the successful execution of research initiatives. We foster international collaboration and contribute to local economic growth in Latin America, addressing key challenges in the Medtech landscape.

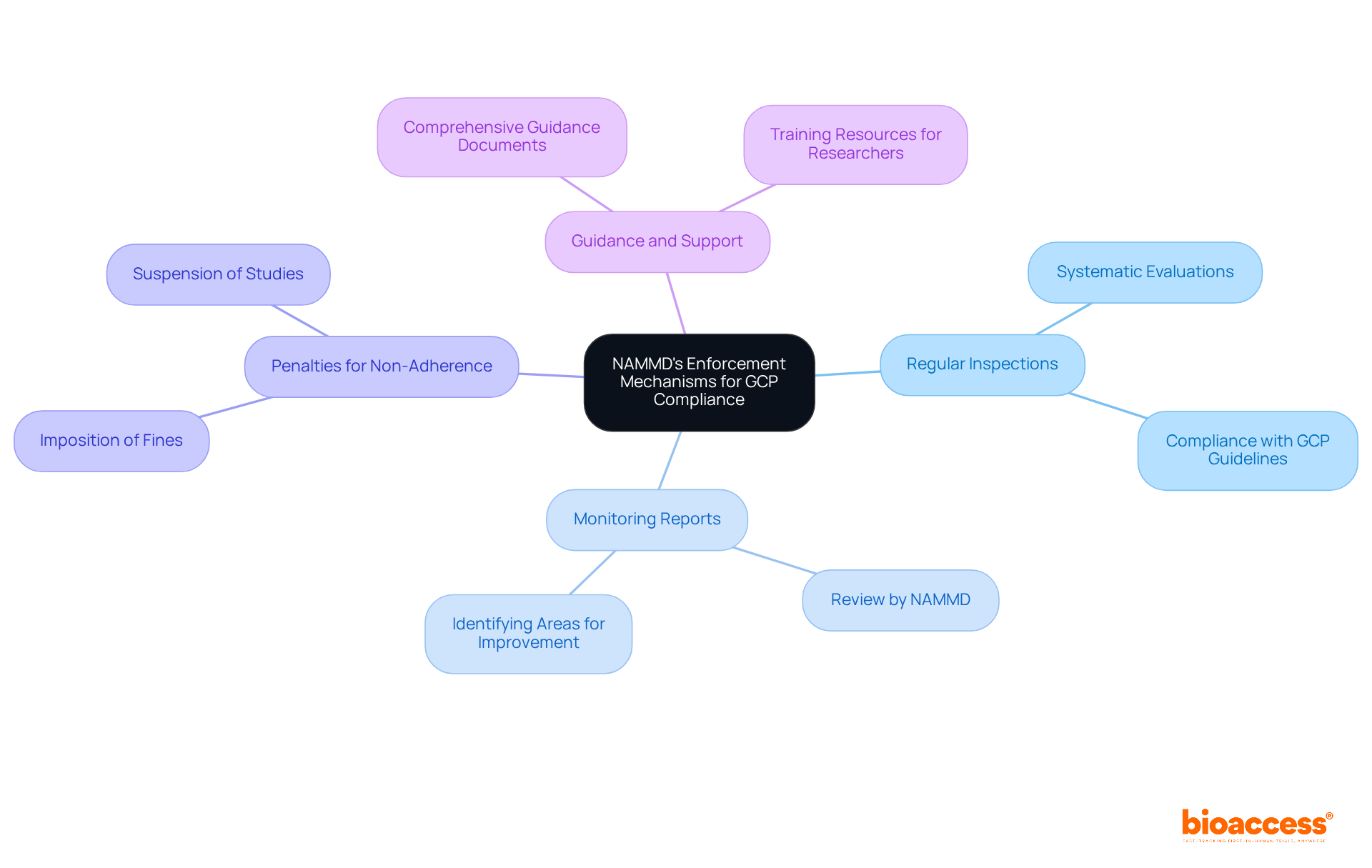
The National Agency for Medicines and Medical Devices (NAMMD) is essential for the good clinical practice enforcement by NAMMD in Romania. This agency's commitment to maintaining high standards in clinical research is underscored by several key enforcement mechanisms:
These mechanisms are vital for upholding the integrity of research studies and ensuring participant safety. It is imperative for researchers to remain vigilant and committed to these regulations, as adherence not only protects participants but also enhances the credibility of the research conducted.
To ensure compliance with GCP, organizations must implement comprehensive training programs that encompass the following essential components:
Preliminary Training: All personnel involved in research studies must complete initial GCP training before engaging in any project. This foundational step is crucial for establishing a solid understanding of GCP principles.
Ongoing Education: Regular refresher courses are necessary to keep staff updated on the latest GCP guidelines and regulatory changes. Continuous learning is vital in the ever-evolving landscape of clinical research.
Role-Specific Training: Customized training should be developed for various roles within the research team, ensuring that each member comprehends their specific responsibilities. Tailored training enhances accountability and effectiveness.
Assessment and Feedback: Implement assessments to evaluate the effectiveness of training programs and gather feedback for continuous improvement. This iterative process is key to refining training efforts and ensuring compliance.
By emphasizing training, organizations can cultivate a culture of adherence, which will ultimately enhance the quality of their clinical studies through good clinical practice enforcement by nammd. The commitment to ongoing education and assessment not only enhances compliance but also drives excellence in research outcomes.

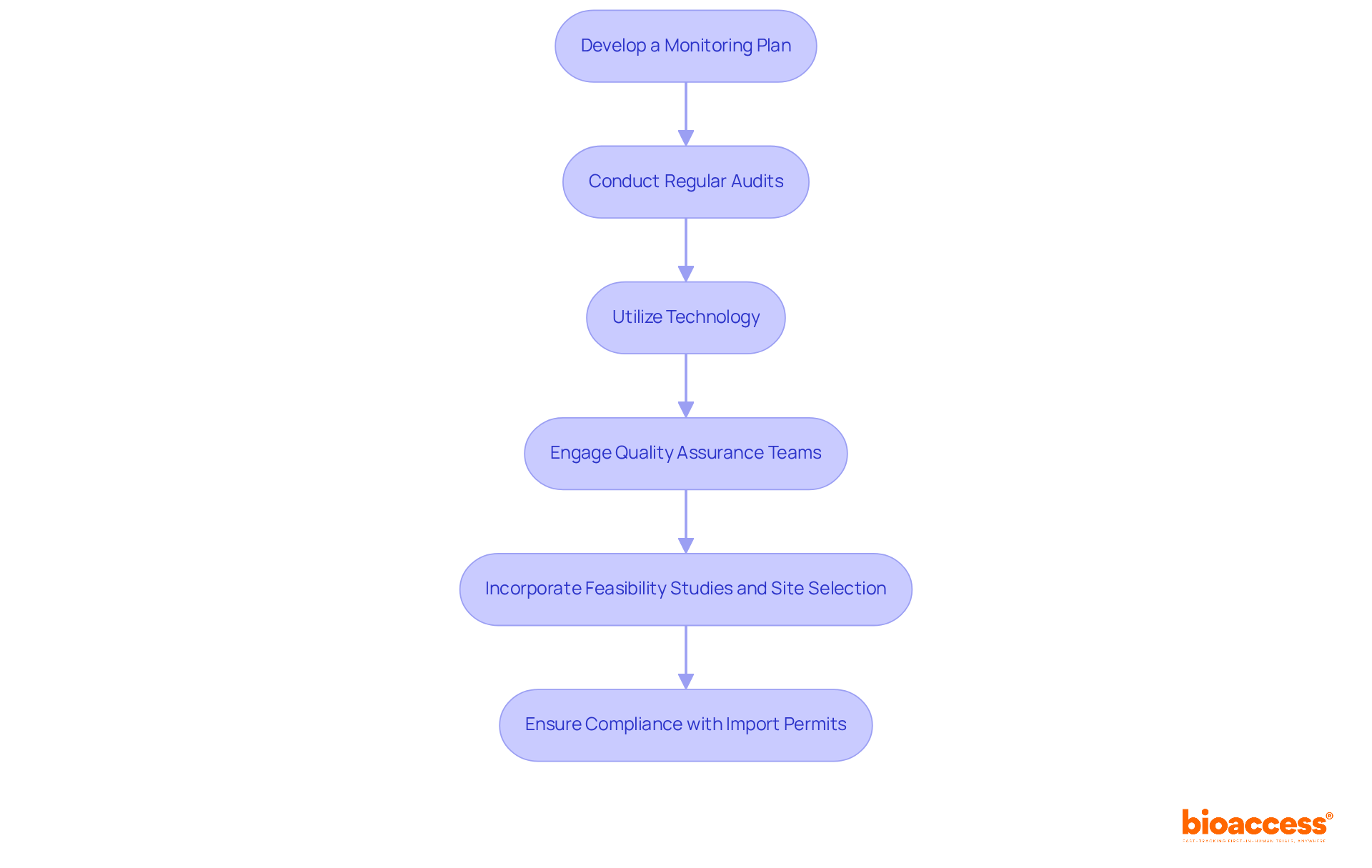
Ongoing monitoring and quality assurance are essential for ensuring good clinical practice enforcement by nammd in clinical research. Organizations like bioaccess must take decisive steps to enhance their processes:
By implementing robust monitoring and quality assurance practices, organizations like bioaccess can significantly enhance the reliability of their trials while safeguarding participant welfare. This proactive approach not only aligns with current technological trends but also reinforces a commitment to ethical conduct in clinical research.
Good clinical practice (GCP) enforcement by NAMMD is crucial for upholding the ethical and scientific integrity of clinical research. By adhering to principles such as ethical conduct, informed consent, data integrity, participant safety, and strict protocol compliance, researchers can cultivate trust and reliability in their studies. This framework not only protects the rights and welfare of participants but also bolsters the credibility of research outcomes.
Key arguments throughout the article underscore NAMMD's role in enforcing GCP through:
The significance of effective training programs cannot be overstated; these initiatives are vital for equipping research personnel with essential knowledge and skills. Furthermore, continuous monitoring and quality assurance practices guarantee that organizations maintain high standards throughout the research process.
Ultimately, the commitment to good clinical practice is a shared responsibility that transcends mere regulatory compliance. By prioritizing ethical standards and participant welfare, researchers can make substantial contributions to advancing medical knowledge while fostering a culture of accountability and excellence in clinical research. Engaging with NAMMD's enforcement mechanisms and investing in training and monitoring will not only elevate the quality of clinical trials but also ensure that the rights of participants remain central to research efforts.
What is good clinical practice (GCP)?
Good clinical practice (GCP) is a framework of internationally accepted ethical and scientific quality standards essential for the design, conduct, recording, and reporting of studies involving human subjects.
What are the core principles of good clinical practice?
The core principles of good clinical practice include ethical conduct, informed consent, data integrity, participant safety, and compliance with protocols.
How does ethical conduct relate to GCP?
Ethical conduct in GCP ensures that trials adhere to principles rooted in the Declaration of Helsinki, prioritizing the rights and welfare of participants.
What is the importance of informed consent in clinical trials?
Informed consent is crucial as it requires participants to receive comprehensive information about the study, enabling them to provide voluntary and informed consent. Failure to secure proper consent can lead to regulatory warnings and jeopardize participant safety and data integrity.
Why is data integrity important in clinical research?
Data integrity is vital because all information gathered during experiments must be accurate, reliable, and verifiable. Documentation deficiencies are a common issue in GCP inspections, highlighting the need for meticulous record-keeping.
What does participant safety entail in the context of GCP?
Participant safety entails ensuring the safety and well-being of study participants as the primary concern throughout the research. Ethical lapses can lead to significant regulatory repercussions.
What is the significance of compliance with protocols in GCP?
Compliance with protocols is mandatory as strict adherence to the approved study protocol is crucial. Deviations from the protocol are often cited as leading deficiencies in inspections.
How does bioaccess® support researchers and sponsors in adhering to GCP?
Bioaccess® provides comprehensive research study management services, including feasibility assessments, site selection, regulatory reviews, study setup, import permits, project management, and reporting, to support the successful execution of research initiatives while ensuring compliance with regulatory standards.