


This article highlights the best practices for mastering human factors testing in medical devices, underscoring its critical role in enhancing usability, safety, and effectiveness. By engaging participants early, conducting iterative evaluations, and adhering to regulatory standards, organizations can significantly reduce user errors and improve patient outcomes.
In the ever-evolving Medtech landscape, understanding these practices is essential for addressing key challenges. The integration of human factors testing not only fosters a safer environment for patients but also enhances the overall effectiveness of medical devices.
As we navigate the complexities of clinical research, collaboration becomes paramount. By implementing these strategies, stakeholders can ensure that their devices meet the highest standards of usability and safety, ultimately leading to better patient care.
Are you ready to elevate your approach to human factors testing? Embrace these practices to drive innovation and improve outcomes in your medical device development.
Human factors testing stands at the intersection of usability and safety in the medical device industry, playing a pivotal role in ensuring that products meet the needs of users while minimizing risks. Regulatory bodies like the FDA emphasize the importance of user-centric design, and manufacturers are increasingly recognizing the value of structured testing methodologies that uncover potential user errors and design flaws. However, with evolving standards and the complexity of human interactions, how can developers effectively navigate this landscape to enhance both compliance and user experience?
This article delves into the best practices for mastering human factors testing, offering insights that can lead to safer, more effective medical devices. By understanding the critical role of human factors testing, stakeholders can better align their products with user needs, ultimately fostering a safer healthcare environment.
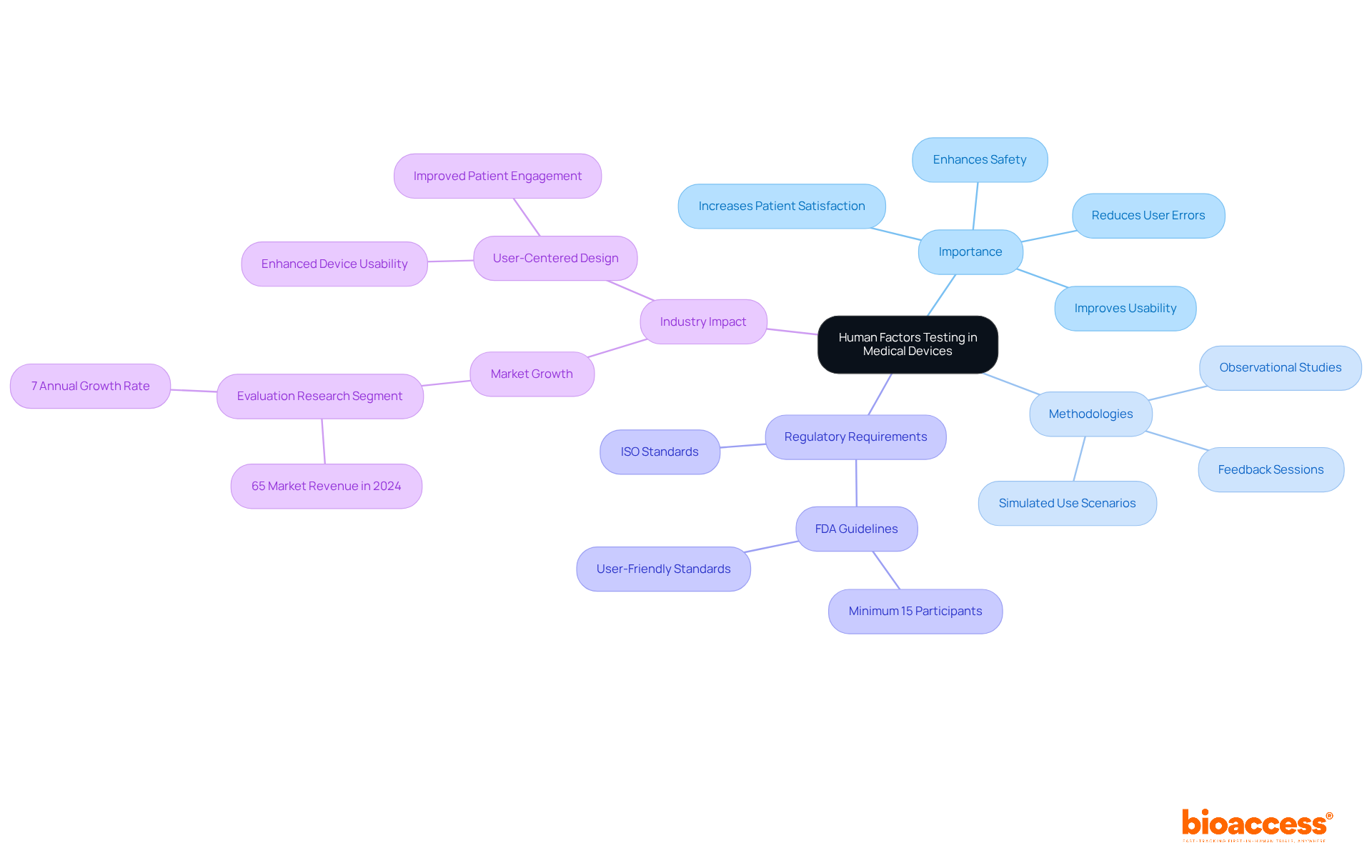
Human factor testing in medical devices is essential for evaluating interactions with individuals, ultimately improving usability, safety, and effectiveness. This process identifies potential user errors and design flaws that could lead to adverse outcomes. Various methodologies, such as simulated use scenarios, observational studies, and feedback sessions, provide valuable insights into user behavior, preferences, and limitations. For instance, a recent study revealed that tools designed with user needs in mind significantly reduce the risk of misuse, thereby improving patient safety and satisfaction.
Regulatory organizations, including the FDA, underscore the importance of human factors evaluation, mandating that products meet user-friendly standards to mitigate use-related risks. The FDA requires a minimum of 15 participants in user assessments, emphasizing the necessity of thorough evaluation. In 2024, the evaluation research sector represented over 65% of the human factors and user experience engineering services market revenue, reflecting the growing recognition of its role in ensuring equipment safety and efficacy.
As industry leaders assert, "Investing in specialized evaluation services is not merely a regulatory requirement - it’s a dedication to improved healthcare results." A case study on the Comparative Ease-of-Use Analysis of Automatic Sensor Applicator illustrates how usability evaluations lead to enhanced device design and increased participant engagement. Overall, implementing human factor testing methods can significantly reduce user errors and bolster patient safety.
To implement effective human factors testing strategies, consider the following best practices:
Define Clear Objectives: Establish specific goals for the evaluation process, such as identifying critical tasks or potential participant errors. Clear objectives direct the creation of the assessment protocol and ensure that the study tackles pertinent user experience issues.
Engage Participants Early: Involve real individuals in the design and testing stages to gather insights on their needs and preferences. Engaging individuals early not only results in more user-focused designs but also increases the chances of recognizing functionality problems that may occur in real-life situations. Research indicates that participant engagement can significantly enhance design outcomes, with studies showing that involving individuals in the early stages can reduce design flaws by up to 30%. Furthermore, the ideal sample size for user evaluation is frequently achieved with as few as 15 to 20 participants, allowing for effective engagement of a representative user group.
Conduct Iterative Evaluation: Utilize an iterative approach to assessment, allowing for multiple rounds of feedback and refinement. This method helps identify issues early, reducing the risk of costly redesigns later. Iterative assessment is crucial; no medical device has successfully passed final usability evaluations without prior iterative reviews, highlighting its significance in the development process. A case study on iterative evaluation illustrates how this cyclical process enhances user satisfaction and overall product performance.
Simulate Real-World Conditions: Create evaluation environments that closely mimic actual use scenarios. This realism aids in revealing functionality problems that may not be obvious in controlled environments. For example, research has indicated that assessments in environments that mimic clinical situations can result in a 25% rise in the detection of functionality issues compared to conventional lab environments.
Document Findings Thoroughly: Maintain detailed records of evaluation procedures, user feedback, and identified issues. Comprehensive documentation is crucial for regulatory submissions and future reference, ensuring that all findings are transparent and traceable. Effective communication of results is vital for the success of human factor testing, particularly for manufacturers seeking regulatory approval. As usability expert Jared Spool points out, 'User evaluation should aim to identify flaws rather than validate existing designs.'
By implementing these strategies, developers can improve the efficacy of their human factors evaluations, resulting in safer and more user-friendly medical devices.

To ensure compliance with regulatory standards in human factors testing, manufacturers must take decisive steps:
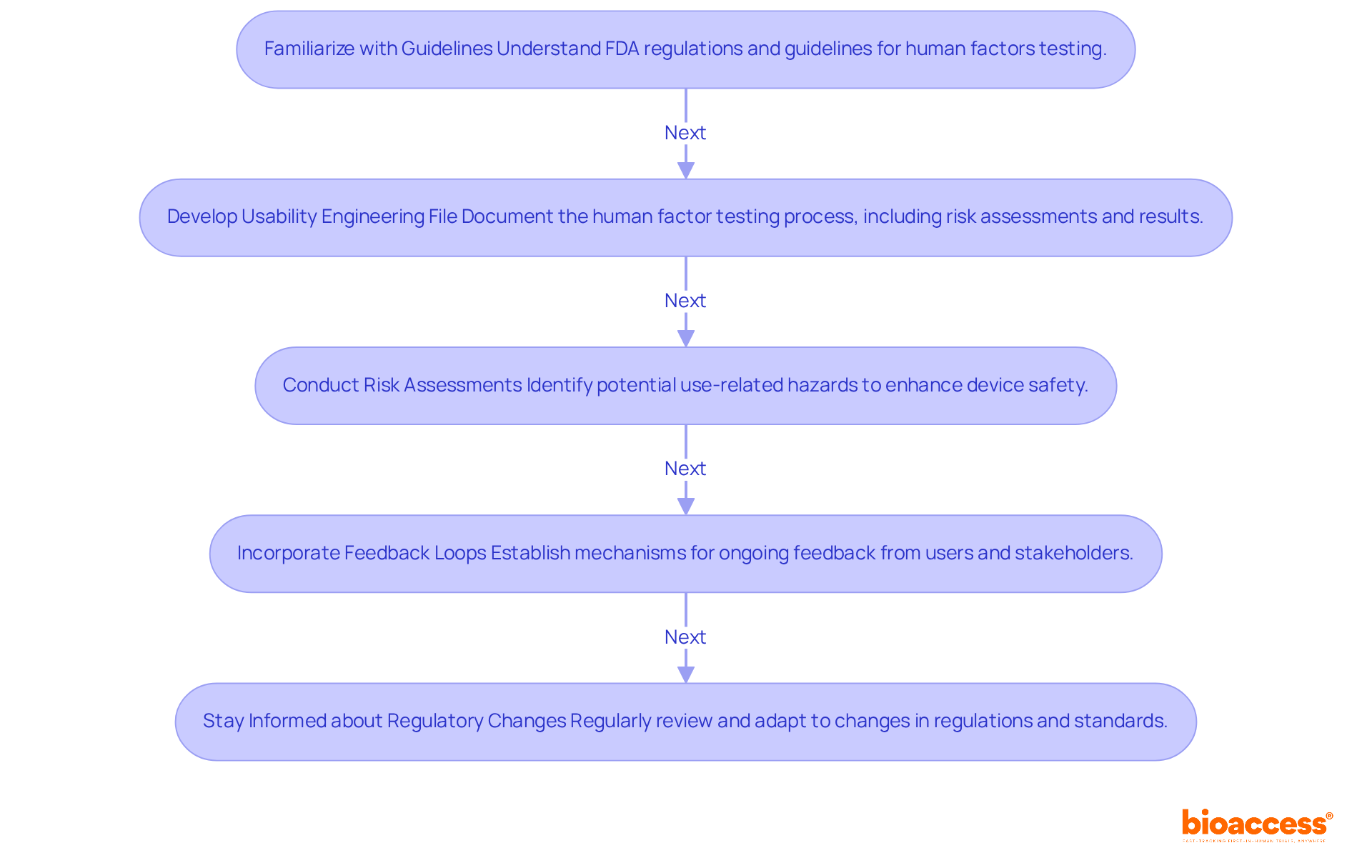
Familiarize with Relevant Guidelines: It's crucial to understand the specific regulations established by the FDA and other regulatory organizations regarding human factors and practical evaluations. Essential documents include the FDA's recommendations on integrating human factor testing and experience engineering into medical devices, underscoring the importance of these components in minimizing human error. Experts like Ana Criado, with her extensive experience in regulatory affairs and biomedical engineering, alongside Katherine Ruiz, a specialist in Regulatory Affairs for Medical Devices and In Vitro Diagnostics in Colombia, can provide invaluable insights into these guidelines based on their practical experiences.
Manufacturers should develop a Usability Engineering File (UEF) that meticulously documents the human factor testing process, including risk assessments, requirements analysis, and testing results. This file is vital for regulatory submissions and must reflect the comprehensive nature of usability engineering, as emphasized by recent FDA updates. Ana's background in health economics can guide the development of a robust UEF that meets regulatory expectations.
Conduct Risk Assessments: Thorough risk assessments are essential to identify potential use-related hazards. Given that approximately 33% of medical device incidents reported to the FDA stem from operator mistakes, incorporating human factor testing into this proactive strategy is crucial for creating safer devices and demonstrating adherence to regulatory standards. Collaborating with regulatory consultants like Ana Criado can enhance the effectiveness of these evaluations by leveraging their expertise in pinpointing key error factors.
Incorporate Feedback Loops: Establishing mechanisms for ongoing feedback from participants and stakeholders throughout the development process is vital. This practice not only supports compliance but also enhances product quality, ensuring that items effectively meet user needs. The expertise of professionals such as Katherine Ruiz can be instrumental in implementing effective feedback strategies that align with regulatory requirements.
Stay Informed about Regulatory Changes: Regularly reviewing and adapting to changes in regulations and standards is necessary to maintain compliance and ensure that evaluation practices remain current. Engaging with regulatory experts can provide insights into developing a robust Usability Engineering File that aligns with evolving guidelines.
By adhering to these compliance strategies, manufacturers can effectively navigate the regulatory landscape, ensuring their products are both safe and market-ready.
Evaluating human factors can significantly enhance satisfaction and contribute to the overall success of medical instruments. Here are the key benefits:
Enhanced Usability: Identifying and addressing usability issues early in the design process allows manufacturers to create devices that are simpler and more intuitive for users. This leads to higher satisfaction rates. It is advisable to conduct at least two to three rounds of formative assessments during product development cycles to facilitate effective design changes.
Effective human factor testing identifies potential user errors, which leads to design modifications that minimize the risk of misuse. This reduction in errors can improve patient outcomes and lower liability risks for manufacturers, aligning with the FDA's guidance on maximizing product safety and effectiveness.
Increased Market Acceptance: User-friendly devices that meet the needs of healthcare professionals and patients are more likely to gain market acceptance, resulting in increased sales and market share. Case studies have demonstrated that user-centered design significantly reduces the likelihood of product recalls due to usability issues.
Improved Training Effectiveness: Well-designed tools often require less instruction for users, conserving time and resources for healthcare establishments. This efficiency can lead to faster adoption of new technologies, as users can interact with the equipment more intuitively.
Positive Brand Reputation: Companies that prioritize human factors in product development tend to be viewed more favorably by consumers and stakeholders, enhancing their reputation in the industry. This positive perception is crucial in a competitive market where trust and reliability are essential.
By recognizing and leveraging these benefits, manufacturers can not only comply with regulatory requirements but also enhance their products through human factor testing, ultimately driving success in the competitive medical device market.

Human factors testing is crucial in the development of medical devices, ensuring products are not only effective but also user-friendly and safe. By prioritizing user interactions and experiences, manufacturers can significantly reduce the risk of errors and enhance overall patient satisfaction. This process transcends mere regulatory compliance; it embodies a commitment to delivering high-quality healthcare solutions that genuinely meet user needs.
The article highlights several best practices for implementing effective human factors testing strategies. Key insights include:
Furthermore, emphasizing compliance with regulatory standards and thorough documentation enables manufacturers to navigate the complex landscape of medical device regulations while ensuring product safety and efficacy.
In summary, integrating human factors testing is essential for advancing medical device design and enhancing user experience. By adopting these practices, manufacturers not only adhere to regulations but also drive innovation and improve patient outcomes. The future of medical devices relies on the ability to understand and address human factors, making it imperative for industry stakeholders to prioritize these evaluations in their development processes.
What is human factors testing in medical devices?
Human factors testing in medical devices evaluates interactions between users and devices to improve usability, safety, and effectiveness by identifying potential user errors and design flaws.
Why is human factors testing important?
It is important because it helps prevent adverse outcomes by enhancing user experience and safety, ultimately leading to better healthcare results.
What methodologies are used in human factors testing?
Methodologies include simulated use scenarios, observational studies, and feedback sessions that provide insights into user behavior, preferences, and limitations.
How does user-centered design impact medical devices?
Tools designed with user needs in mind significantly reduce the risk of misuse, thereby improving patient safety and satisfaction.
What regulatory requirements exist for human factors testing?
Regulatory organizations like the FDA mandate that products meet user-friendly standards and require a minimum of 15 participants in user assessments to ensure thorough evaluation.
What does the market for human factors and user experience engineering services look like?
In 2024, the evaluation research sector represented over 65% of the market revenue for human factors and user experience engineering services, indicating its growing importance.
What is the perspective of industry leaders on human factors evaluation?
Industry leaders view investing in specialized evaluation services as a commitment to improved healthcare results, beyond just regulatory compliance.
Can you provide an example of human factors testing in action?
A case study on the Comparative Ease-of-Use Analysis of an Automatic Sensor Applicator demonstrated how usability evaluations can lead to enhanced device design and increased participant engagement.