


The article underscores the critical importance of mastering ISO 14971 for successful clinical trials, as it offers a structured approach to risk management for medical devices. This mastery is essential for ensuring both patient safety and regulatory compliance. A detailed explanation of the risk management process supports this assertion, highlighting key components such as:
Each of these elements plays a vital role in identifying and mitigating potential hazards associated with medical devices throughout their lifecycle.
Understanding the complexities of risk management is crucial in the realm of clinical trials, where the stakes are high and patient safety is paramount. ISO 14971 serves as a foundational framework, guiding organizations in systematically identifying and mitigating risks associated with medical devices. By delving into the principles of ISO 14971, this article explores how its effective implementation not only enhances regulatory compliance but also significantly improves clinical outcomes. The challenge remains: how can clinical trial managers seamlessly integrate these risk management strategies into their existing processes to ensure both safety and efficacy?
ISO risk management 14971 serves as the cornerstone for managing hazards in medical instruments, delineating essential procedures such as threat analysis, assessment, and control. Its implementation is vital for the success of clinical trials, as it systematically identifies potential hazards associated with medical devices and establishes a robust framework for iso risk management 14971 to manage these risks. Notably, organizations like bioaccess®, recognized as Latin America’s leading CRO, have effectively integrated iso risk management 14971 into their development processes. This integration has resulted in enhanced regulatory compliance and improved patient welfare outcomes during trials, facilitated by services such as feasibility studies and compliance reviews.
Expert opinions underscore the significance of iso risk management 14971 in clinical environments, emphasizing that adherence to iso risk management 14971 not only meets regulatory requirements but also prioritizes patient well-being. A thorough benefit-risk assessment, which is part of iso risk management 14971, is crucial for making informed decisions regarding the design and use of medical instruments. This evaluation ensures that potential benefits are weighed against hazards, fostering a culture of safety and accountability in clinical trials. By embedding iso risk management 14971 principles into their operational frameworks, organizations like bioaccess® can significantly mitigate risks, ultimately leading to safer medical products and more favorable clinical outcomes.
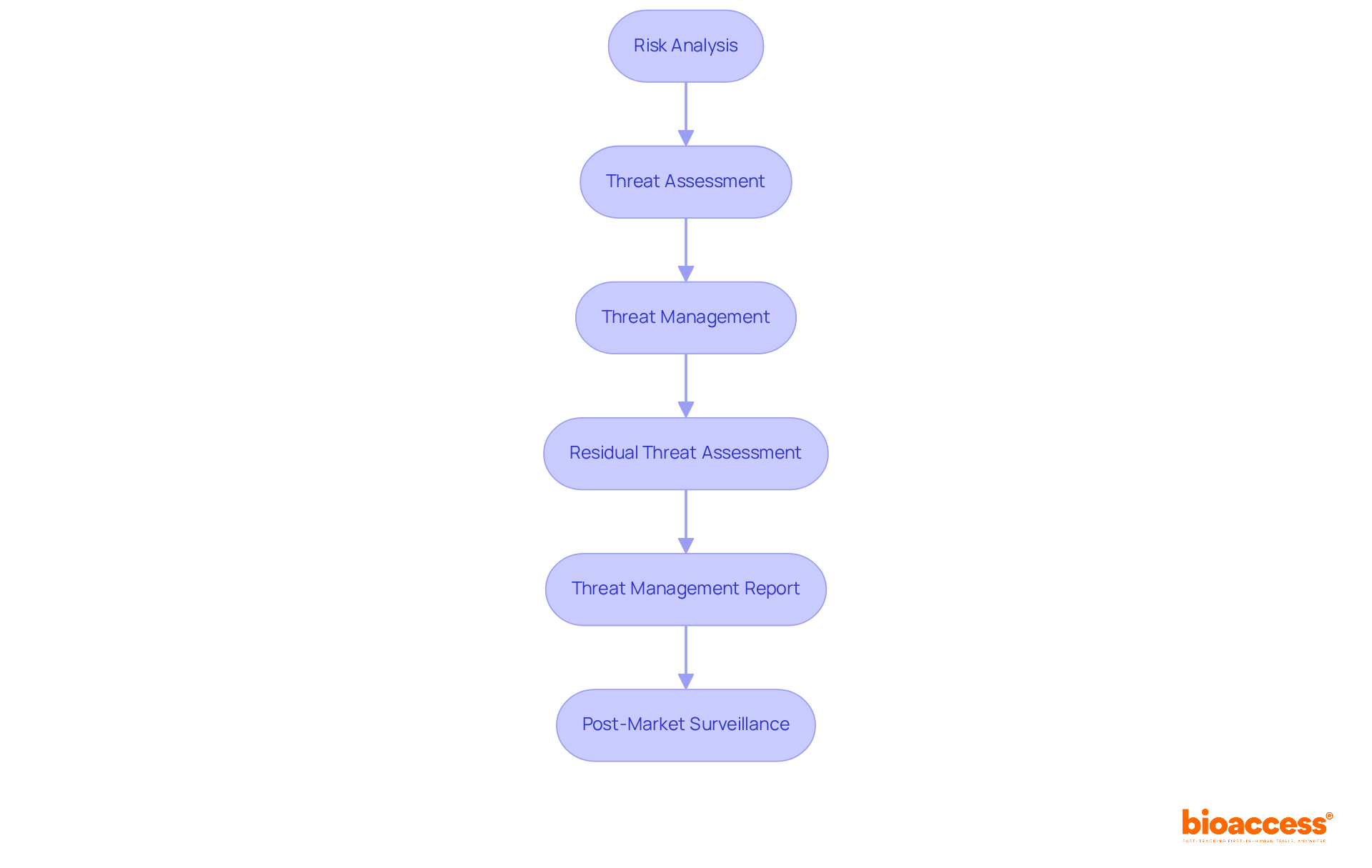
Applying the iso risk management 14971 procedure is essential for ensuring the safety and efficacy of medical instruments. This process encompasses several key steps that are critical for clinical research success:
Risk Analysis: Begin by identifying potential hazards associated with the medical device. Evaluate these dangers by considering both the probability of occurrence and the seriousness of possible harm. This foundational step is vital for aligning user needs with design inputs.
Threat Assessment: Assess the acceptability of identified hazards based on predefined criteria. This involves contrasting estimated threats against acceptable thresholds set by the organization, ensuring that all hazards are systematically evaluated.
Threat Management: Implement measures to mitigate identified hazards. This may involve redesigning the apparatus, incorporating protective features, or providing clear warnings and instructions for use. Effective hazard management is crucial for enhancing device safety.
Residual Threat Assessment: After applying control measures, evaluate the remaining dangers to ensure they remain acceptable. This step is critical for confirming that all potential hazards have been adequately addressed.
Threat Management Report: Document the entire threat management process, including analysis, evaluation, and control measures. This report serves as a vital reference for regulatory submissions and audits, ensuring compliance with iso risk management 14971 standards.
Post-Market Surveillance: Continuously monitor the product's performance in the market to identify any new hazards that may arise. This ongoing process is essential for maintaining security and effectiveness throughout the product lifecycle.
By adhering to these procedures, clinical trial managers can systematically address uncertainties, ensuring compliance with iso risk management 14971 while enhancing the safety and effectiveness of medical products. The typical duration required for assessment in clinical trials may vary, but establishing a robust oversight framework can significantly streamline this process, ultimately leading to quicker and more effective clinical studies.
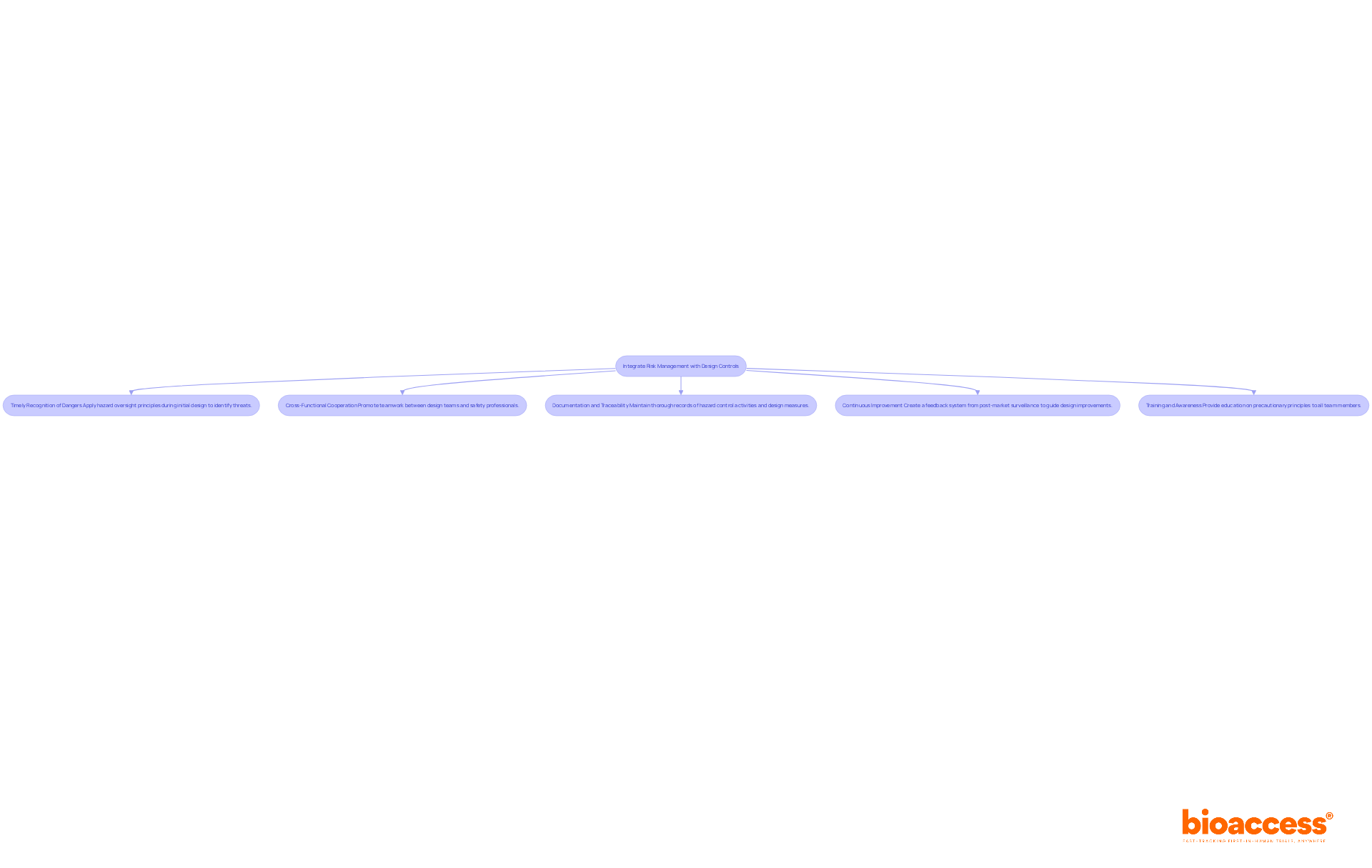
Incorporating hazard oversight with design controls is essential for creating safe and effective medical devices. This integration ensures that safety considerations are embedded throughout the product lifecycle, from initial design to post-market surveillance. Key strategies for achieving this integration include:
Timely Recognition of Dangers: Apply hazard oversight principles during the initial design stage to proactively identify possible threats. This method promotes the development of safer products by tackling issues before they intensify. Statistics show that more than 1.7 million injuries and 83,000 fatalities in the U.S. may be linked to healthcare equipment, highlighting the essential requirement for strong safety protocols.
Cross-Functional Cooperation: Promote teamwork between design teams and safety professionals to effectively integrate protective features into device designs. Consistent communication encourages the recognition and addressing of possible issues early in the development process. As Bijan Elahi points out, strong hazard oversight goes beyond merely adhering to ISO risk management 14971 standards, highlighting the necessity for practical strategies for success.
Documentation and Traceability: Keep thorough records of hazard control activities and design measures. This documentation should clearly outline how hazards were identified, evaluated, and mitigated, ensuring compliance with regulatory requirements. Utilizing a traceability matrix can enhance clarity and organization in the design process, linking design inputs to outputs effectively.
Continuous Improvement: Create a feedback system where information from post-market surveillance guides design improvements and updates to safety practices. This repetitive procedure is crucial for enhancing equipment security over time.
Training and Awareness: Offer education for all team members engaged in the design and development process, highlighting the significance of precautionary principles in ensuring equipment security. Addressing common pitfalls, such as miscommunication due to varying terminology related to design inputs, can further enhance the effectiveness of these training efforts.
By embedding ISO risk management 14971 within design controls, organizations can foster a culture of safety that enhances all aspects of medical device development, ultimately improving patient outcomes and ensuring compliance with regulatory standards.
The implementation of ISO 14971 is imperative for guaranteeing the safety and effectiveness of medical devices in clinical trials. This standard offers a structured approach to risk management, empowering organizations to identify, assess, and mitigate potential hazards associated with medical instruments. By adhering to these principles, companies can not only meet regulatory requirements but also prioritize patient well-being, ultimately resulting in successful clinical trial outcomes.
Key arguments presented in the article underscore the necessity of a comprehensive risk management process. This encompasses:
Furthermore, integrating risk management with design controls enhances safety by embedding precautionary measures throughout the product lifecycle, fostering a culture of safety and accountability within organizations.
Given the significant implications of ISO 14971 on clinical trial success, it is essential for stakeholders in the medical device industry to prioritize its principles. By doing so, they can contribute to the development of safer medical products, improve patient outcomes, and ensure compliance with evolving regulatory standards. Embracing these best practices not only enhances the credibility of clinical trials but also reinforces the commitment to safeguarding patient health and safety within the medical field.
What is ISO 14971?
ISO 14971 is a standard that provides a framework for managing risks associated with medical devices, including procedures for threat analysis, assessment, and control.
Why is ISO 14971 important for clinical trials?
ISO 14971 is vital for clinical trials as it systematically identifies potential hazards related to medical devices and establishes a robust framework for managing these risks, which enhances regulatory compliance and patient welfare outcomes.
How have organizations like bioaccess® implemented ISO 14971?
Organizations like bioaccess® have effectively integrated ISO 14971 into their development processes, resulting in improved regulatory compliance and better patient welfare during clinical trials through services such as feasibility studies and compliance reviews.
What is the significance of benefit-risk assessment in ISO 14971?
The benefit-risk assessment is crucial in ISO 14971 as it helps in making informed decisions about the design and use of medical instruments by weighing potential benefits against hazards, promoting safety and accountability in clinical trials.
How does adherence to ISO 14971 affect patient well-being?
Adherence to ISO 14971 prioritizes patient well-being by ensuring that risks are managed effectively, ultimately leading to safer medical products and more favorable clinical outcomes.