


Understanding the complex landscape of clinical research in Bulgaria requires a thorough examination of local ethics committees (LECs) and their timelines and Standard Operating Procedures (SOPs). Researchers can gain critical insights into how these committees uphold ethical standards and prioritize participant welfare, which ultimately influences the success of their studies.
However, securing the necessary approvals presents significant challenges, including potential delays and intricate documentation requirements.
How can researchers effectively navigate these obstacles to ensure their projects not only meet regulatory standards but also excel in a competitive field?
The authorization of research studies in Bulgaria relies on local ethics committee timelines and SOPs in Bulgaria to ensure that ethical standards are upheld and the rights and welfare of participants are protected. Each LEC operates under specific guidelines and comprises professionals from diverse fields, including medicine, ethics, and law. To initiate a clinical study, researchers must secure not only the consent of the Bulgarian Drug Agency (BDA) but also the endorsement of a local ethics committee, which evaluates the ethical dimensions of the proposed research based on local ethics committee timelines and SOPs in Bulgaria.
Understanding the role of local ethics committee timelines and SOPs in Bulgaria is vital for researchers, as it highlights the significance of ethical adherence and the necessity of obtaining their consent before commencing any clinical study. LECs meticulously assess the scientific credibility of the research, the adequacy of informed consent procedures, and the potential risks in relation to the benefits of the study, in accordance with local ethics committee timelines and SOPs in Bulgaria. The BDA aims for a review duration of just 35 days for trial submissions, highlighting the efficiency of the regulatory system in Bulgaria.
Moreover, a comprehensive collection of documents is required by the BDA, including the study protocol and informed consent forms, to facilitate the ethical review process. The Clinical Trials Information System (CTIS) further streamlines submissions and reduces review times, contributing to a more efficient regulatory environment. This collaborative framework not only enhances the integrity of clinical research but also fosters trust among stakeholders.
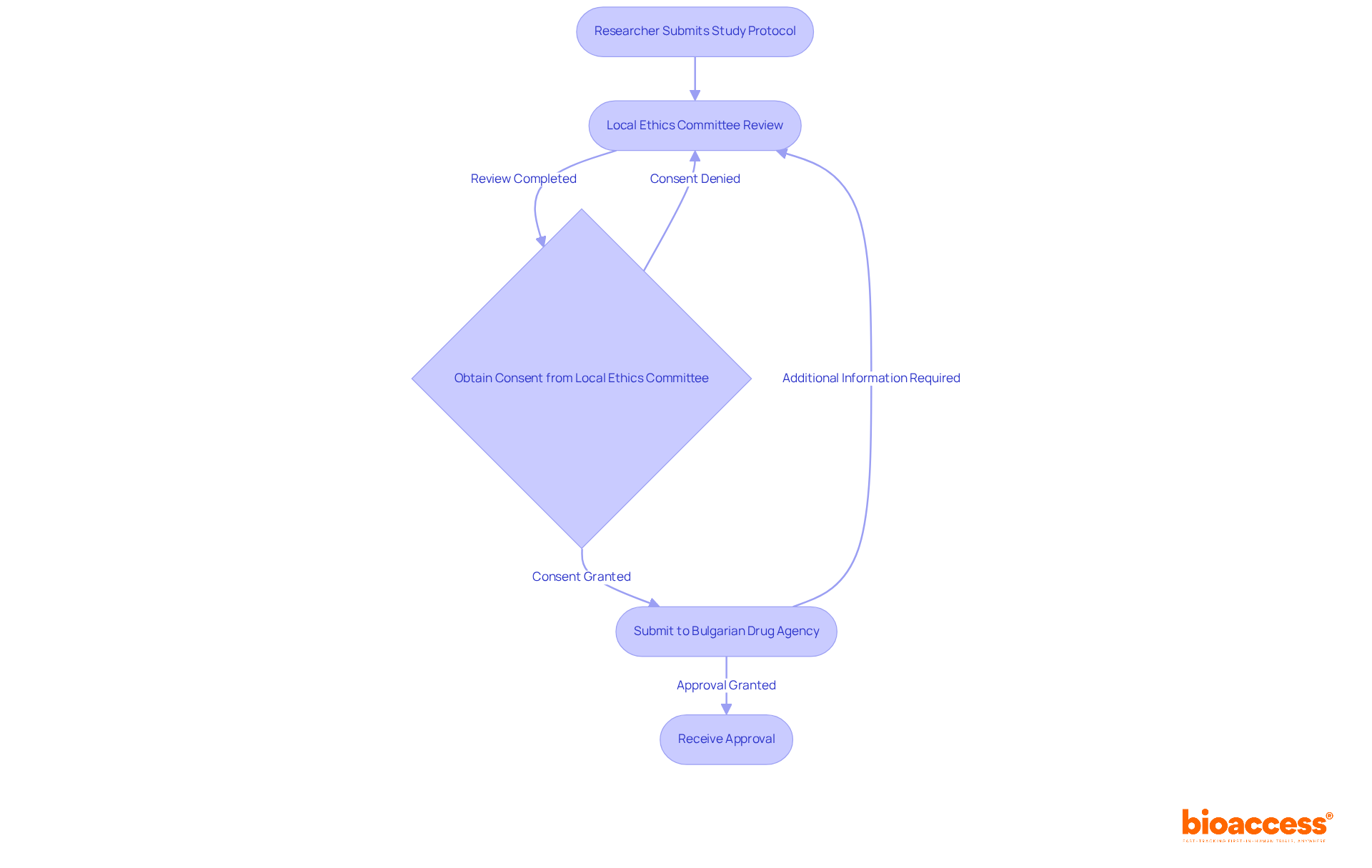
In Bulgaria, the local ethics committee timelines and SOPs typically require about 30 days for review after submission to obtain consent. This timeline may extend if the committee seeks additional information. Incorporating local ethics committee timelines and SOPs into your clinical trial planning is essential, as delays in ethics approval can significantly impact the overall study schedule. Furthermore, the Bulgarian Drug Agency (BDA) mandates a separate evaluation process, which also takes around 30 days. Therefore, effective coordination of submissions to both the local ethics committee timelines and SOPs and the BDA is crucial to minimize delays.
With approximately 550 active research studies currently underway in Bulgaria, understanding local ethics committee timelines and SOPs is essential for maintaining competitive enrollment and adhering to project schedules. Katherine Ruiz, an expert in regulatory affairs for medical devices and in vitro diagnostics, offers valuable insights into navigating these processes. Bioaccess® provides comprehensive management services for research studies, including:
This ensures that your research studies are conducted efficiently and effectively.

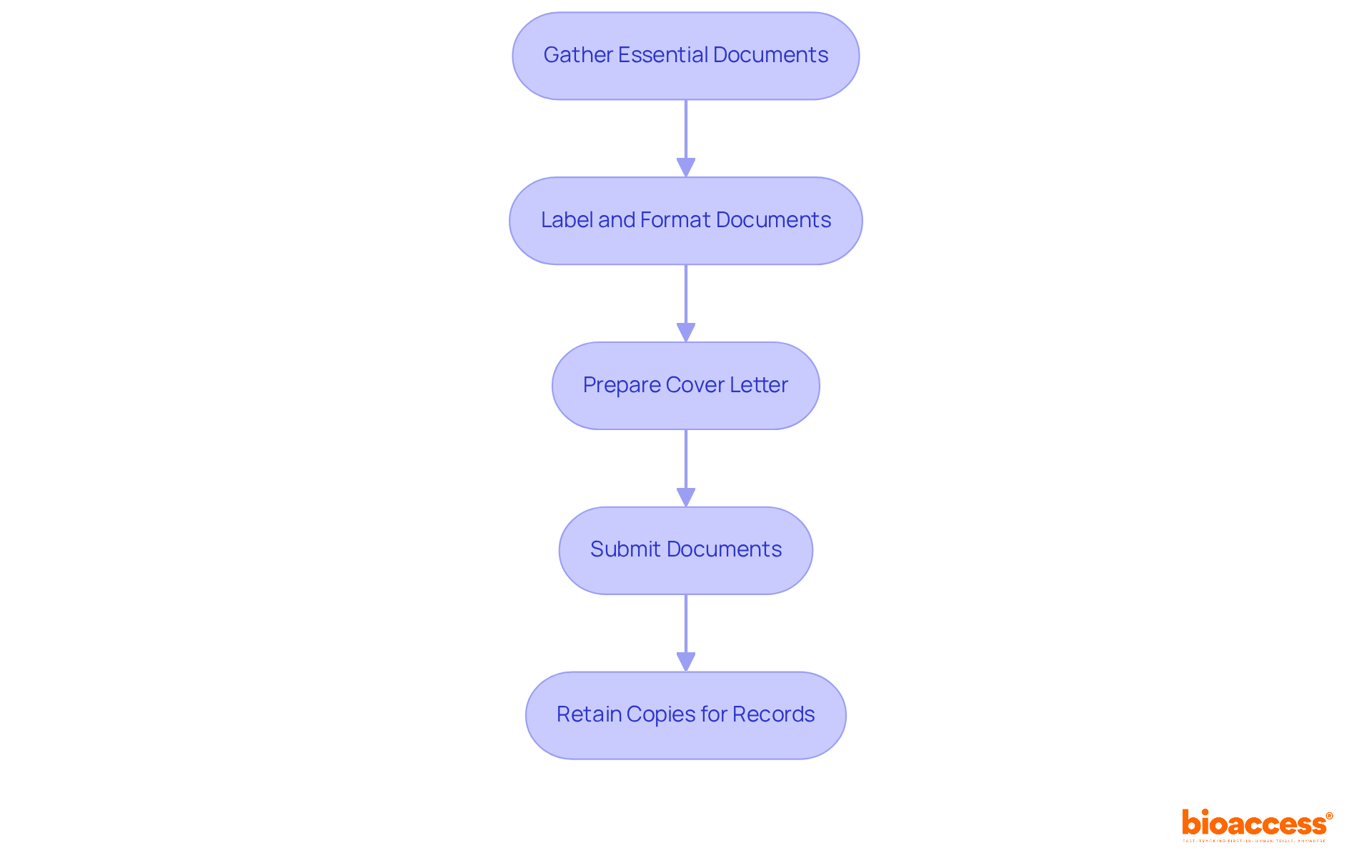
To ensure a successful submission to the local ethics committee timelines and SOPs in Bulgaria, researchers must adhere to specific Standard Operating Procedures (SOPs). Begin by gathering all essential documents, such as the study protocol, informed consent forms, and relevant safety data. Each document should be meticulously labeled and formatted according to the committee's guidelines. A cover letter in Bulgarian is also crucial; it should succinctly summarize the submission's purpose and list all enclosed documents.
Once the documents are prepared, submit them through the designated channels, ensuring you retain copies for your records. Following these SOPs significantly enhances the chances of receiving prompt approval, as demonstrated by the local ethics committee timelines and SOPs in Bulgaria, which include a 60-day evaluation period for research studies. Furthermore, it's essential to note that initial medical research applications must be submitted through the Clinical Trials Information System (CTIS), a requirement that took effect on January 31, 2023. This organized approach not only simplifies the submission process but also improves the overall effectiveness of research activities in the region.
As Tatyana Benisheva emphasizes, research studies provide additional treatment options and health resources for healthcare systems, underscoring the importance of a well-executed submission method. By following these guidelines, researchers can navigate the complexities of the submission process with confidence.
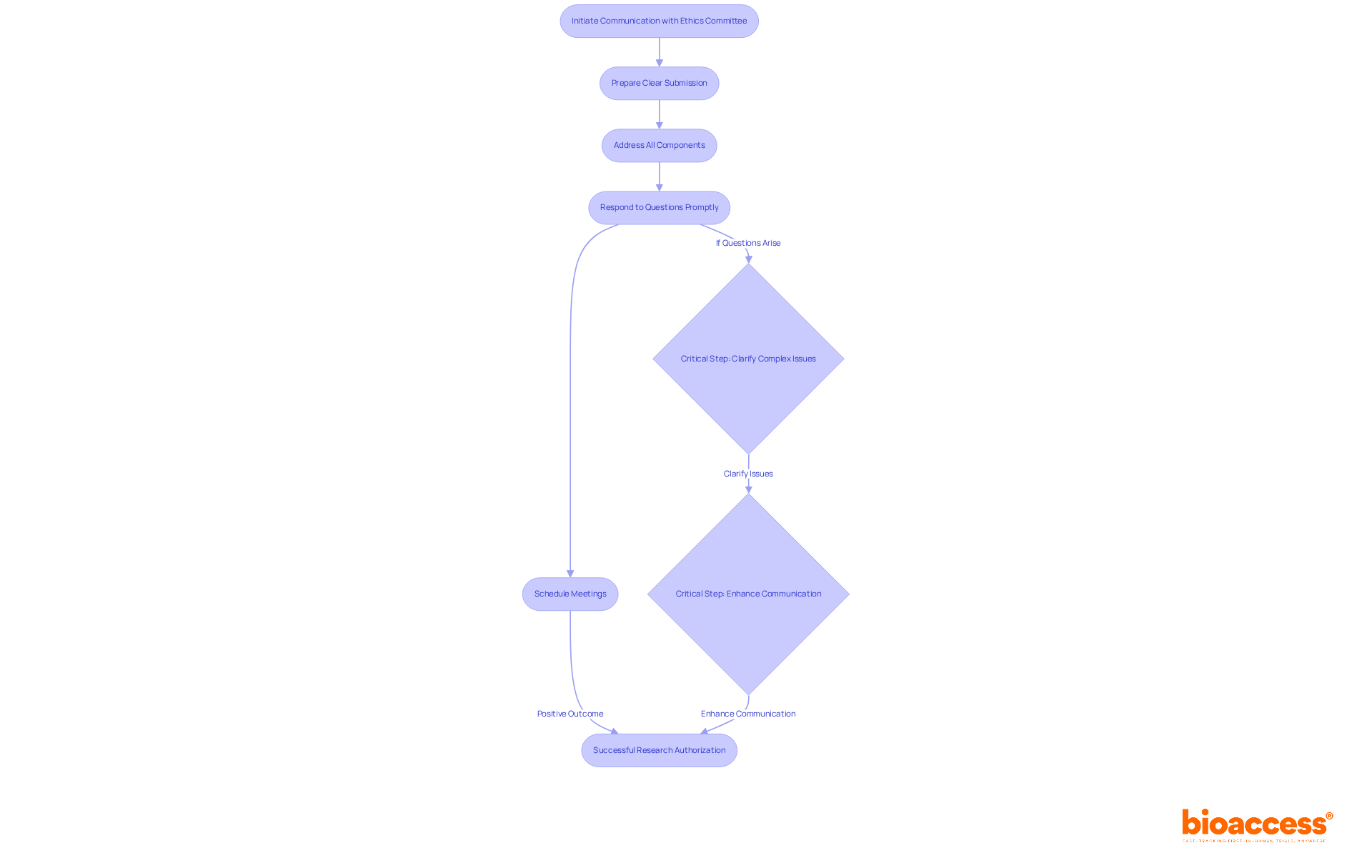
Establishing a solid relationship with the local ethics committee timelines and SOPs in Bulgaria is crucial for streamlining the authorization steps in research studies. Clear and concise submissions are vital; ensure that your application is well-organized and addresses all necessary components. When the committee raises questions or concerns, respond promptly and comprehensively.
Engaging in direct communication - such as scheduling meetings or calls with committee members - can clarify complex issues and demonstrate your commitment to ethical standards. This proactive approach not only fosters a collaborative relationship but also significantly enhances the likelihood of favorable outcomes. Statistics indicate that applications characterized by thorough communication and engagement enjoy a higher acceptance rate, underscoring the importance of these interactions.
Successful instances from Bulgarian clinical trials illustrate that researchers who actively interact with local ethics committee timelines and SOPs in Bulgaria frequently encounter more streamlined procedures and shortened timelines, ultimately aiding the progress of their studies. By prioritizing these relationships, you position your research for success.
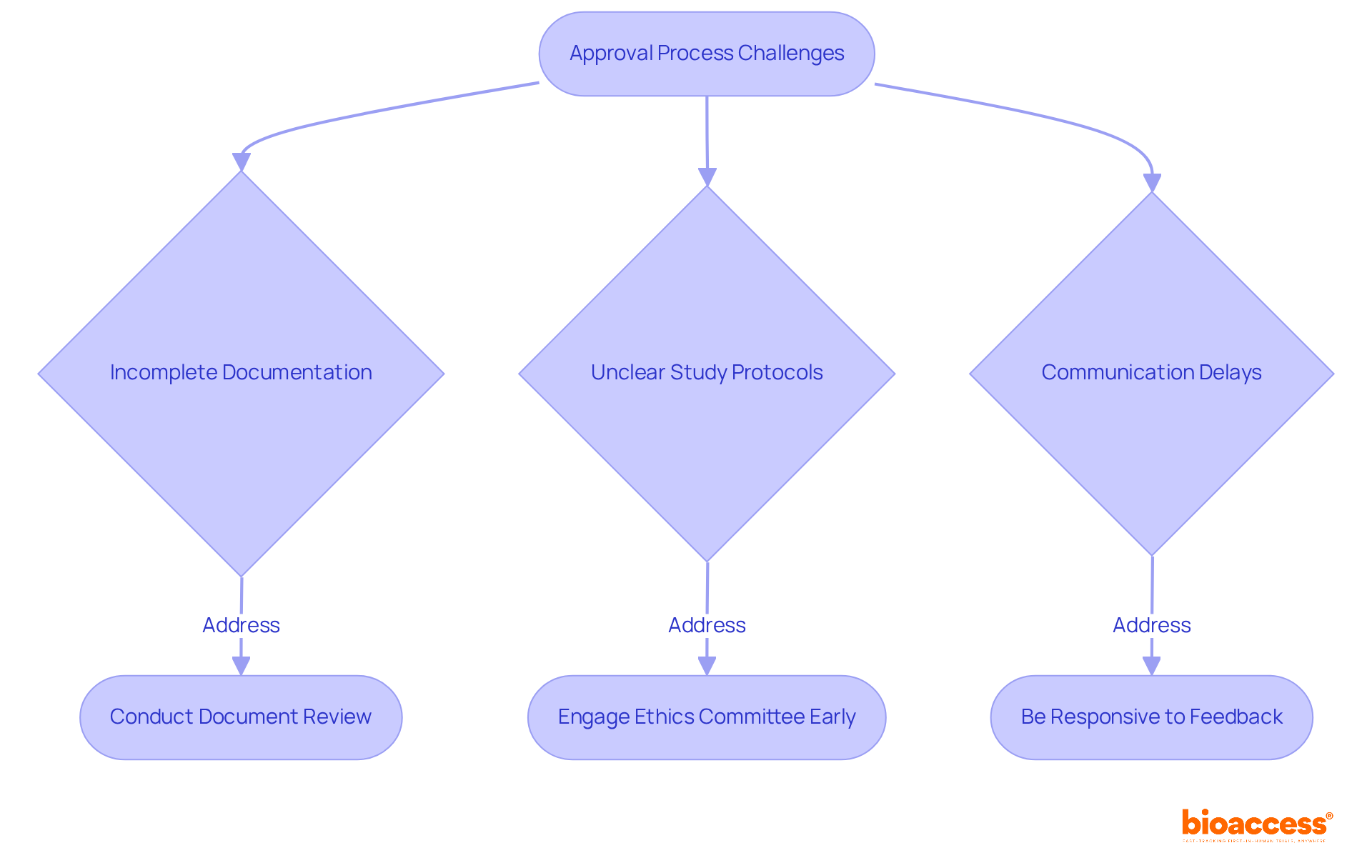
Navigating the approval procedure with local ethics committee timelines and SOPs in Bulgaria presents significant challenges that may hinder clinical research. Incomplete documentation, unclear study protocols, and communication delays are common obstacles. Notably, statistics reveal that around 30% of these delays arise from insufficient documentation, emphasizing the critical need for meticulous preparation.
To address these issues effectively, conducting a comprehensive review of all documents before submission is essential. Engaging the ethics committee early in the process can clarify their requirements and expectations, significantly reducing the likelihood of misunderstandings. As Allison Dunn aptly noted, "Consistently aligning actions with your stated values is the clearest sign of ethical leadership." This observation underscores the importance of ethical considerations in navigating these complexities.
Moreover, being responsive to feedback and inquiries for additional information can expedite the acceptance timeline concerning local ethics committee timelines and SOPs in Bulgaria. By anticipating potential challenges and fostering open lines of communication, researchers can minimize delays and enhance the efficiency of the approval process. This proactive approach not only streamlines operations but also reinforces the commitment to ethical standards in clinical research.
Understanding the complexities of local ethics committee timelines and Standard Operating Procedures (SOPs) in Bulgaria is crucial for any researcher aiming to conduct clinical studies. The focus on ethical standards and participant welfare not only protects the integrity of research but also cultivates a collaborative environment between researchers and regulatory bodies. By prioritizing these elements, researchers can navigate the approval process with increased confidence and efficiency.
This article outlines essential components of the ethics approval process, including critical timelines for obtaining consent from local ethics committees and the Bulgarian Drug Agency. It underscores the significance of thorough documentation and effective communication, which are vital for overcoming common challenges that may arise during the approval process. Furthermore, insights from industry experts highlight the advantages of adhering to established SOPs and maintaining open lines of communication with ethics committee members.
In conclusion, researchers are urged to adopt a proactive approach when engaging with local ethics committees. By comprehending the timelines, meticulously following SOPs, and fostering clear communication, the likelihood of successful study approvals rises significantly. This commitment not only enhances the efficiency of research activities but also strengthens the ethical foundation upon which clinical research is built, ultimately contributing to advancements in healthcare and participant safety.
What is the role of local ethics committees (LECs) in Bulgaria regarding research studies?
Local ethics committees in Bulgaria are responsible for evaluating the ethical dimensions of proposed research studies, ensuring that ethical standards are upheld, and protecting the rights and welfare of participants.
What is required to initiate a clinical study in Bulgaria?
Researchers must obtain consent from the Bulgarian Drug Agency (BDA) and endorsement from a local ethics committee, which assesses the study's ethical considerations.
How long does the local ethics committee review process typically take in Bulgaria?
The local ethics committee generally requires about 30 days for review after submission to obtain consent, although this timeline may extend if additional information is requested.
What documents are needed for the ethical review process by the BDA?
A comprehensive collection of documents, including the study protocol and informed consent forms, is required by the BDA to facilitate the ethical review process.
What is the review duration for trial submissions by the Bulgarian Drug Agency (BDA)?
The BDA aims for a review duration of approximately 35 days for trial submissions.
How can delays in ethics approval affect clinical trials in Bulgaria?
Delays in ethics approval can significantly impact the overall study schedule, making it crucial for researchers to incorporate local ethics committee timelines into their planning.
What is the significance of the Clinical Trials Information System (CTIS) in Bulgaria?
The CTIS streamlines submissions and reduces review times, contributing to a more efficient regulatory environment for clinical research.
What services does Bioaccess® provide for managing research studies in Bulgaria?
Bioaccess® offers services such as feasibility assessments, site selection, compliance evaluations, study setup, import permits, project management, and reporting to ensure efficient and effective conduct of research studies.