


The essential compliance steps for clinical research directors under MDR 2017/745 are pivotal:
Adherence to these steps is not merely a regulatory requirement; it is critical for ensuring participant safety and achieving regulatory compliance. This, in turn, enhances the quality and success of clinical trials. Therefore, it is imperative for clinical research directors to prioritize these actions to foster a robust research environment.
Navigating the intricate landscape of medical device regulation is no small feat, particularly with the implementation of MDR 2017/745 by the European Union. This regulation establishes a high standard for safety and efficacy, mandating rigorous compliance measures that clinical research directors must master to ensure successful outcomes.
As the stakes rise, the challenge becomes evident: how can research leaders effectively align their practices with these stringent requirements while safeguarding participant safety and advancing innovation?
This article explores essential compliance steps, strategies for overcoming obstacles, and the critical implications of medical device classification, providing a roadmap for clinical research directors striving for excellence in their studies.
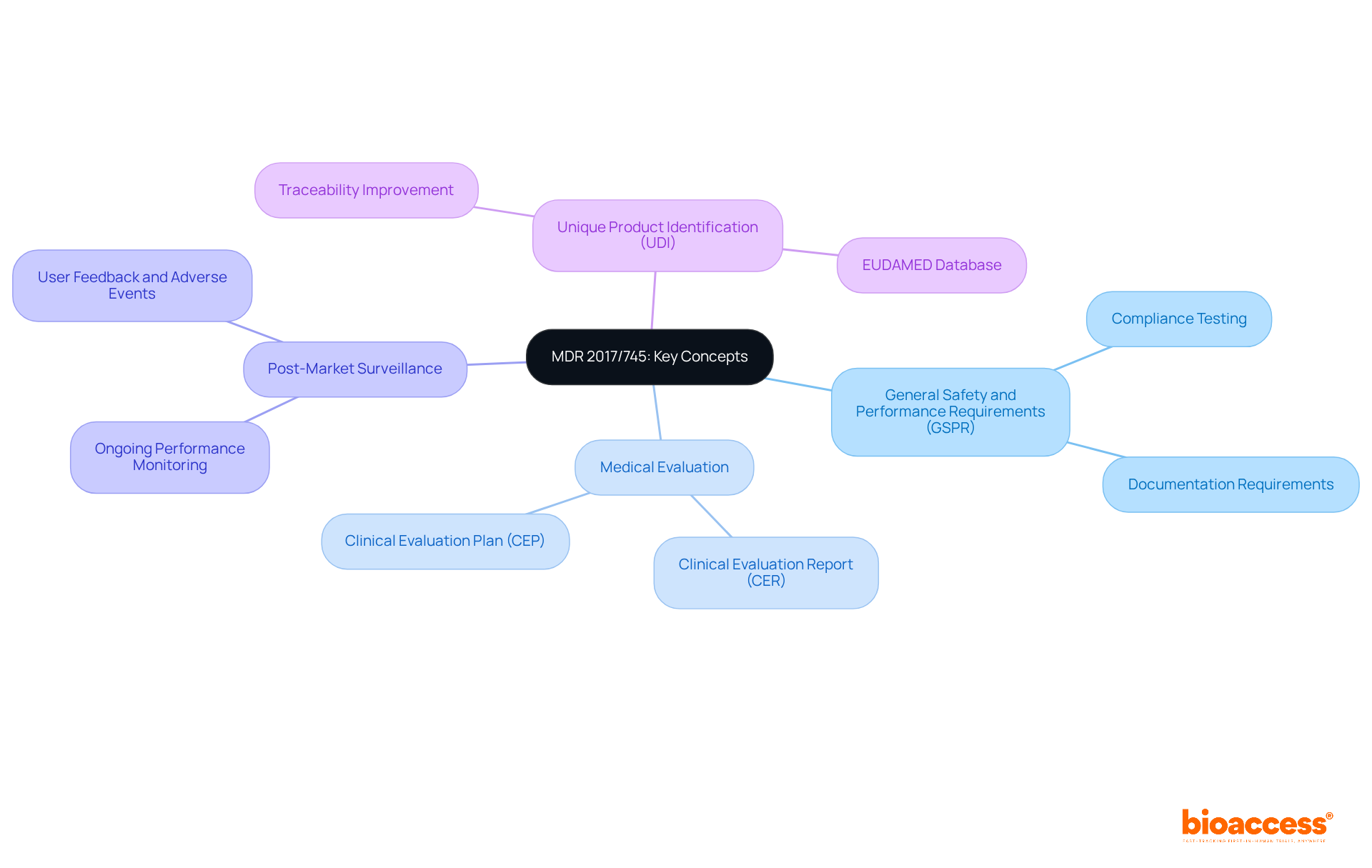
The regulation known as MDR 2017/745 establishes a robust legislative framework by the European Union to govern the clinical investigation and market access of medical instruments. Understanding this regulation is crucial for research directors in healthcare as they navigate the complexities of compliance and aim for successful research studies.
Key concepts include:
The emphasis of MDR 2017/745 on rigorous evaluation and ongoing monitoring reflects a commitment to patient safety and device efficacy, making adherence to these regulations paramount in the medical device industry. Collaboration and proactive engagement with these regulatory frameworks will enhance the effectiveness of clinical research and ultimately contribute to improved patient outcomes.
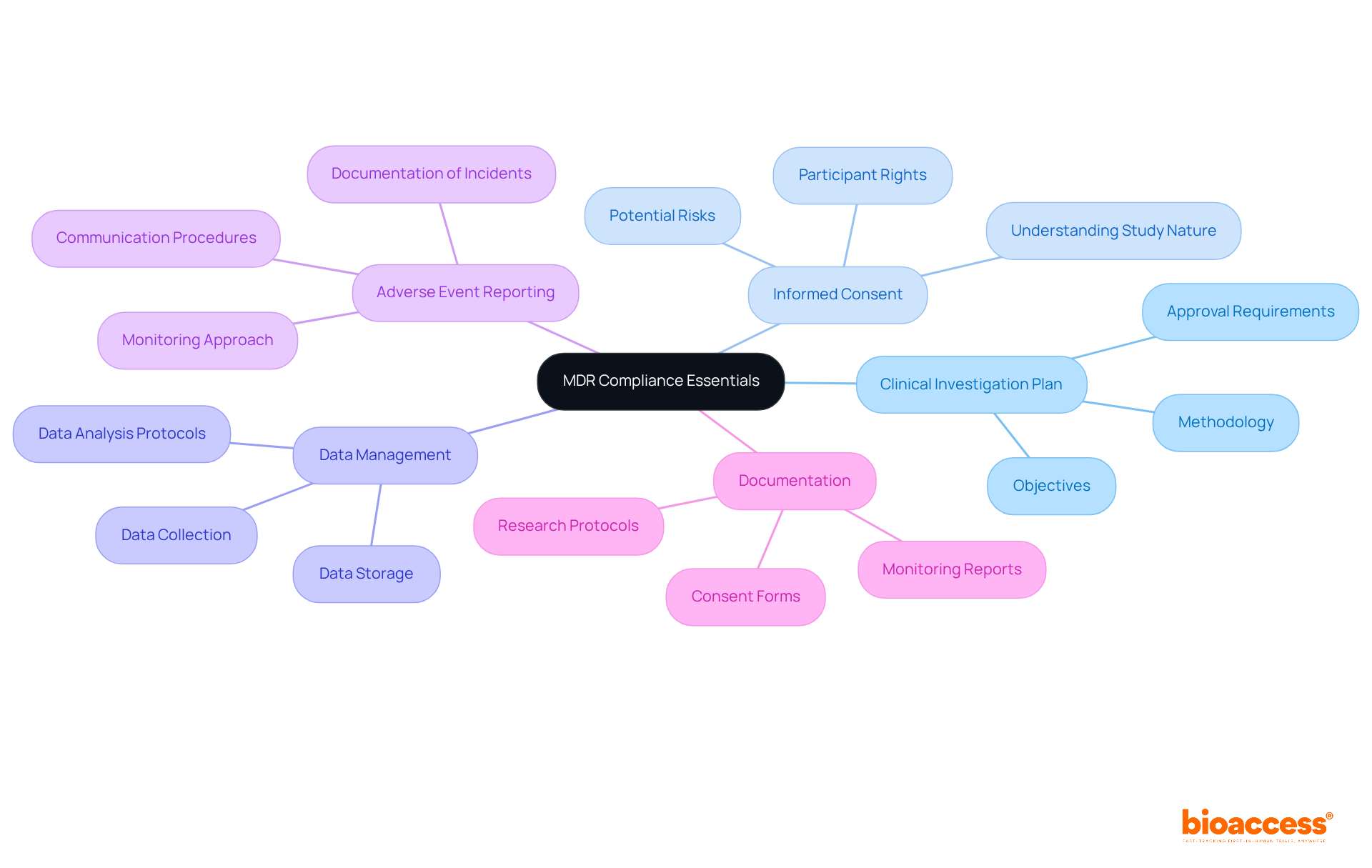
To ensure compliance with MDR 2017/745 during clinical trials, clinical research directors must prioritize several essential elements:
Clinical Investigation Plan (CIP): This comprehensive document outlines the trial's objectives, methodology, statistical considerations, and organizational structure. It is imperative that the CIP receives approval from relevant ethics committees and regulatory authorities, as this is a cornerstone of compliance. Bioaccess offers expertise in reviewing and providing feedback on study documents to enhance the quality of the CIP.
Informed Consent: Obtaining informed consent is crucial. Participants must fully understand the study's nature, potential risks, and their rights before enrollment. Effective communication strategies should be employed to facilitate this understanding, ensuring that consent is truly informed. Bioaccess's extensive management services for research studies include creating informed consent procedures that address various participant requirements.
Data Management: Implementing robust data management practices is vital for maintaining the integrity and confidentiality of clinical data. This encompasses all aspects of data handling, including collection, storage, and analysis protocols, which must adhere to regulatory standards. Bioaccess guarantees that data management practices comply with regulatory standards, improving the reliability of study outcomes.
Adverse Event Reporting: Establishing a systematic approach for monitoring and reporting adverse events is essential for participant safety and regulatory compliance. This encompasses prompt documentation and communication of any incidents that take place during the testing. Bioaccess offers project management and monitoring services that enable efficient adverse event reporting, ensuring participant safety throughout the study.
Documentation: Maintaining comprehensive documentation is necessary to show adherence during audits and inspections. This encompasses research protocols, consent forms, and monitoring reports, all of which must be carefully arranged and easily accessible. Bioaccess assists in maintaining organized documentation, ensuring that all necessary records are available for review.
Following these compliance essentials is vital for the successful implementation of research studies under the MDR 2017/745, ensuring that the rights and safety of participants are maintained while satisfying regulatory requirements. With Bioaccess's extensive capabilities in clinical trial management, research directors can navigate these complexities effectively.
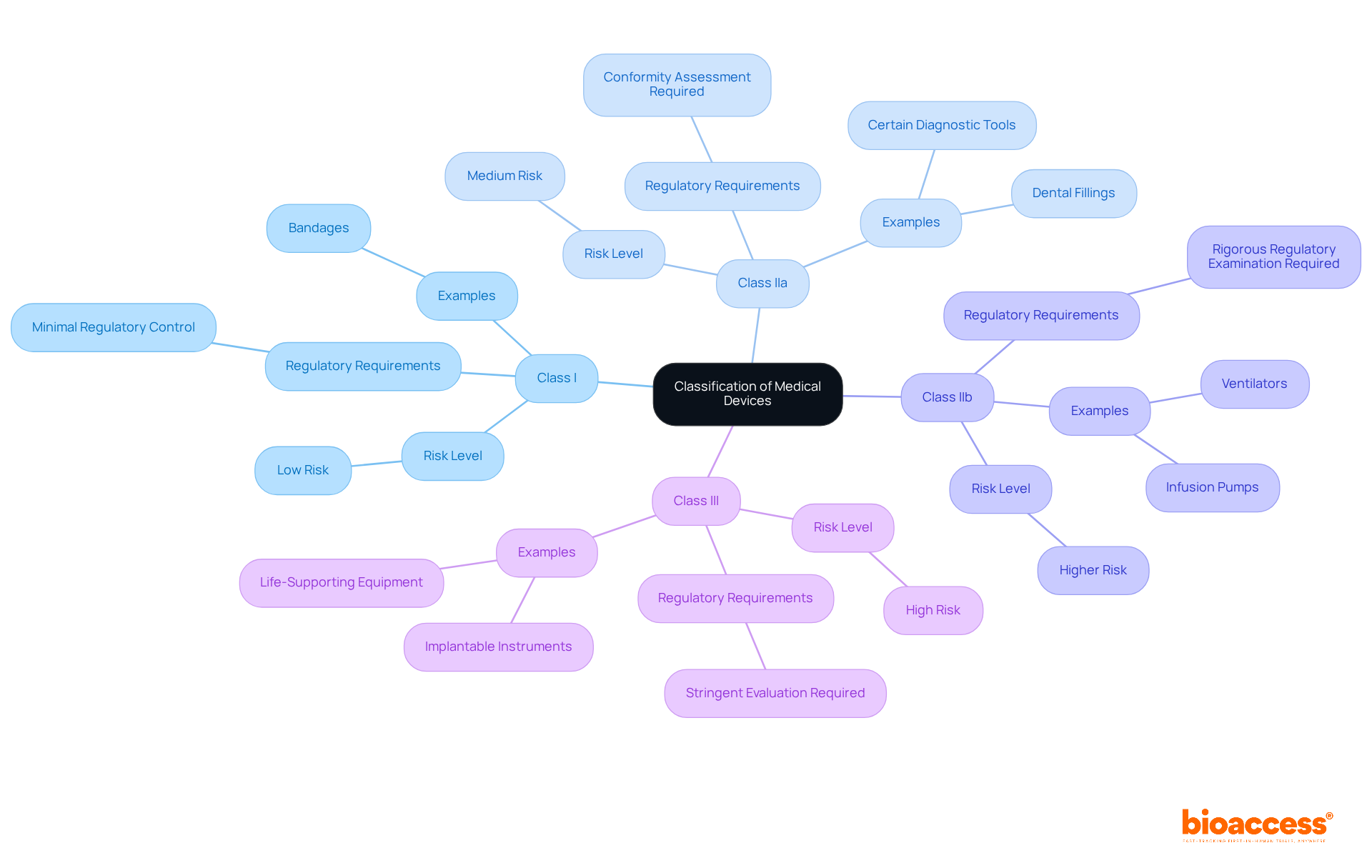
Medical devices are classified under MDR 2017/745 into four main categories based on their risk level:
The classification of a medical device directly influences the regulatory pathway for research involving human subjects. This encompasses the extent of evidence required, the type of pre-market authorization needed, and the responsibilities for post-market monitoring. Understanding these classifications is crucial for research directors, enabling them to tailor their research and development strategies effectively.
At bioaccess®, we offer comprehensive management services for research studies, which include feasibility assessments, site selection, regulatory reviews, setup, import permits, project oversight, and reporting. Our unique sprint approach enables regulatory approval in just 6-8 weeks, a significant reduction compared to the typical 6-12 months seen in the US and EU. This expedited process allows us to enroll treatment-naive cardiology or neurology groups 50% faster than Western locations, effectively addressing regulatory challenges in early-stage trials. Additionally, we collaborate with INVIMA and leverage the expertise of Katherine Ruiz, a specialist in regulatory matters for medical devices and in vitro diagnostics in Colombia, ensuring compliance and streamlining processes.
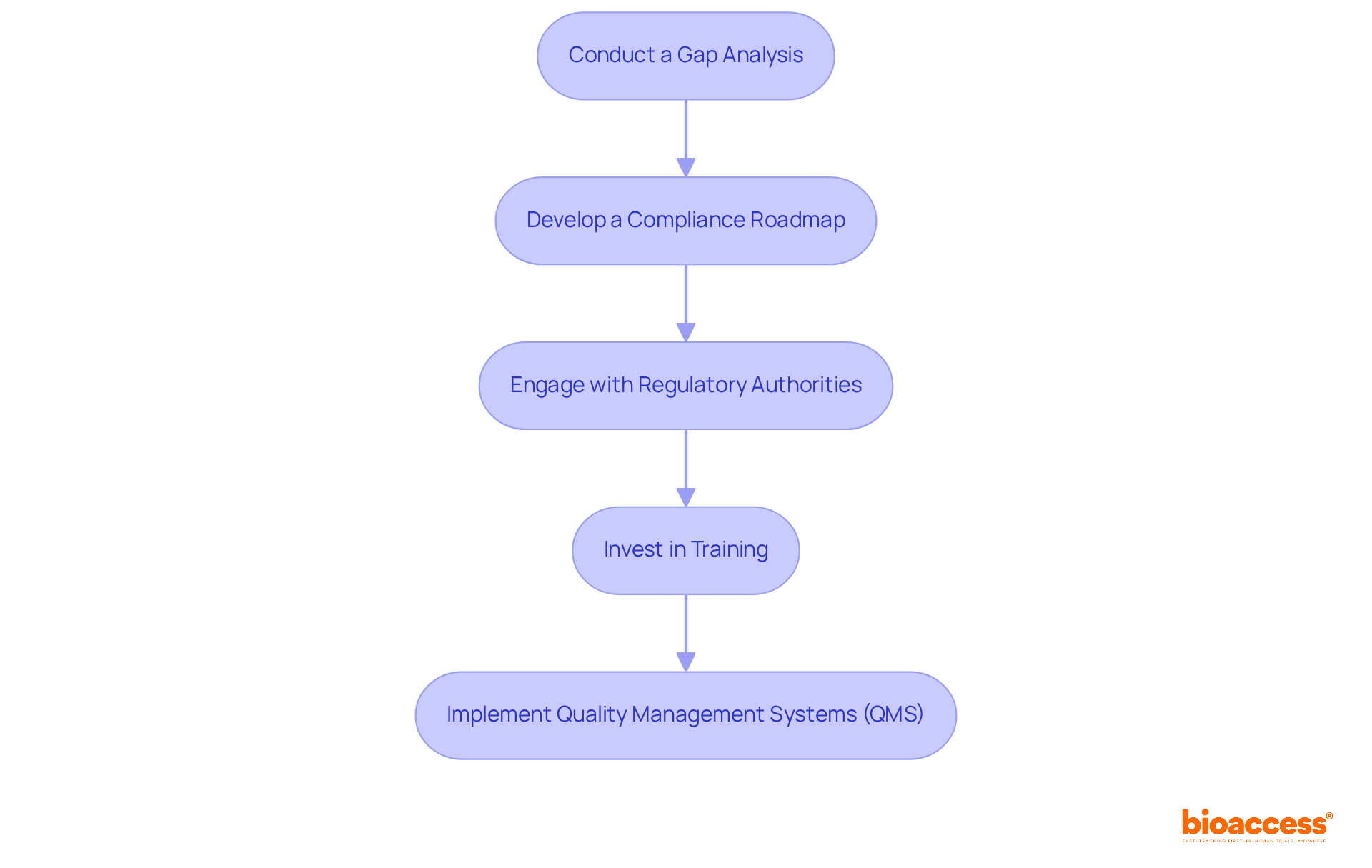
To effectively prepare for compliance with MDR 2017/745, clinical research directors must adopt a strategic approach that encompasses several key initiatives.
Conduct a Gap Analysis: It is essential to assess current practices against MDR requirements to pinpoint areas needing improvement. This analysis should encompass documentation, processes, and team training.
Develop a Compliance Roadmap: A detailed plan must be created, outlining the steps necessary to achieve conformity. This roadmap should include timelines, responsibilities, and resource allocation to ensure clarity and direction.
Engage with Regulatory Authorities: Establishing open lines of communication with regulatory bodies is crucial. This engagement will help clarify requirements and provide guidance on effective adherence strategies.
Invest in Training: Continuous education for staff on MDR 2017/745 requirements and best practices in research is vital. This investment ensures that everyone is equipped to meet regulatory standards effectively.
Implement Quality Management Systems (QMS): Implementing QMS that align with MDR 2017/745 requirements is imperative for development and maintenance. This system should guarantee that all processes are documented, monitored, and continuously improved.
By adopting these strategies, clinical research directors can significantly enhance their preparedness for MDR compliance, ultimately leading to more successful clinical trials.
Mastering MDR 2017/745 is crucial for clinical research directors who must navigate the complex landscape of medical device regulation. This regulation not only highlights the significance of safety and efficacy in medical devices but also establishes a comprehensive framework that guides compliance throughout the clinical research process. Understanding and implementing the key components of MDR 2017/745 is essential for achieving successful research outcomes and safeguarding patient welfare.
The article underscores several critical aspects of MDR 2017/745, including:
By adhering to these compliance essentials and leveraging the support of expert services like Bioaccess, research directors can streamline their processes and enhance the quality of their studies.
Ultimately, the commitment to MDR 2017/745 compliance not only facilitates regulatory approval but also fosters a culture of safety and accountability within the clinical research landscape. As the medical device industry continues to evolve, proactive engagement with these regulations will be crucial. Clinical research directors are encouraged to embrace these compliance strategies and invest in their teams' training, ensuring they are well-equipped to meet the challenges of MDR 2017/745 head-on. By doing so, they can significantly contribute to the advancement of medical technology and improve patient outcomes in the healthcare sector.
What is MDR 2017/745?
MDR 2017/745 is a regulation established by the European Union that provides a legislative framework for the clinical investigation and market access of medical instruments.
Why is understanding MDR 2017/745 important for research directors in healthcare?
Understanding MDR 2017/745 is crucial for research directors as it helps them navigate compliance complexities and aim for successful research studies.
What are the General Safety and Performance Requirements (GSPR) under MDR 2017/745?
GSPR are essential requirements that ensure medical devices are safe and effective, requiring manufacturers to demonstrate compliance through extensive testing and thorough documentation for market approval.
What is the purpose of Medical Evaluation in the context of MDR 2017/745?
Medical Evaluation is a structured procedure that assesses the safety and effectiveness of medical instruments based on health-related information, forming the basis for acquiring market approval.
What is a Clinical Evaluation Report (CER)?
A Clinical Evaluation Report (CER) is a document that summarizes the clinical data and findings for each medical instrument, as mandated by MDR 2017/745.
What does Post-Market Surveillance entail?
Post-Market Surveillance involves the ongoing observation of a product's performance after its release to uphold safety standards and identify any potential issues that may arise during use.
What is Unique Product Identification (UDI) and its significance?
UDI is a system that assigns a unique identifier to each medical product, enhancing traceability and supporting post-market surveillance, ensuring manufacturers maintain accurate records and comply with regulations.
How does MDR 2017/745 contribute to patient safety and device efficacy?
The regulation emphasizes rigorous evaluation and ongoing monitoring, reflecting a commitment to patient safety and device efficacy, making adherence to these regulations essential in the medical device industry.