


Pediatric drug trials stand as a crucial frontier in medical research, yet they remain significantly underexplored, even though children make up a substantial portion of the patient population. Alarmingly, only a small fraction of clinical studies focus on young patients, highlighting an urgent need to develop safe and effective medications specifically tailored for children. As researchers in Montenegro navigate the intricate regulatory landscape for pediatric drug trial authorization, they encounter numerous challenges that could impede progress.
How can these dedicated professionals effectively overcome these barriers to ensure that the unique health needs of children are adequately addressed in clinical research?
Pediatric medication studies are vital for developing safe and effective treatments tailored for children. Given their unique physiological and developmental characteristics, children metabolize medications differently than adults. This distinction underscores the necessity of conducting studies specifically for this demographic. Such research not only ensures appropriate dosing but also rigorously assesses the safety of medications intended for younger patients.
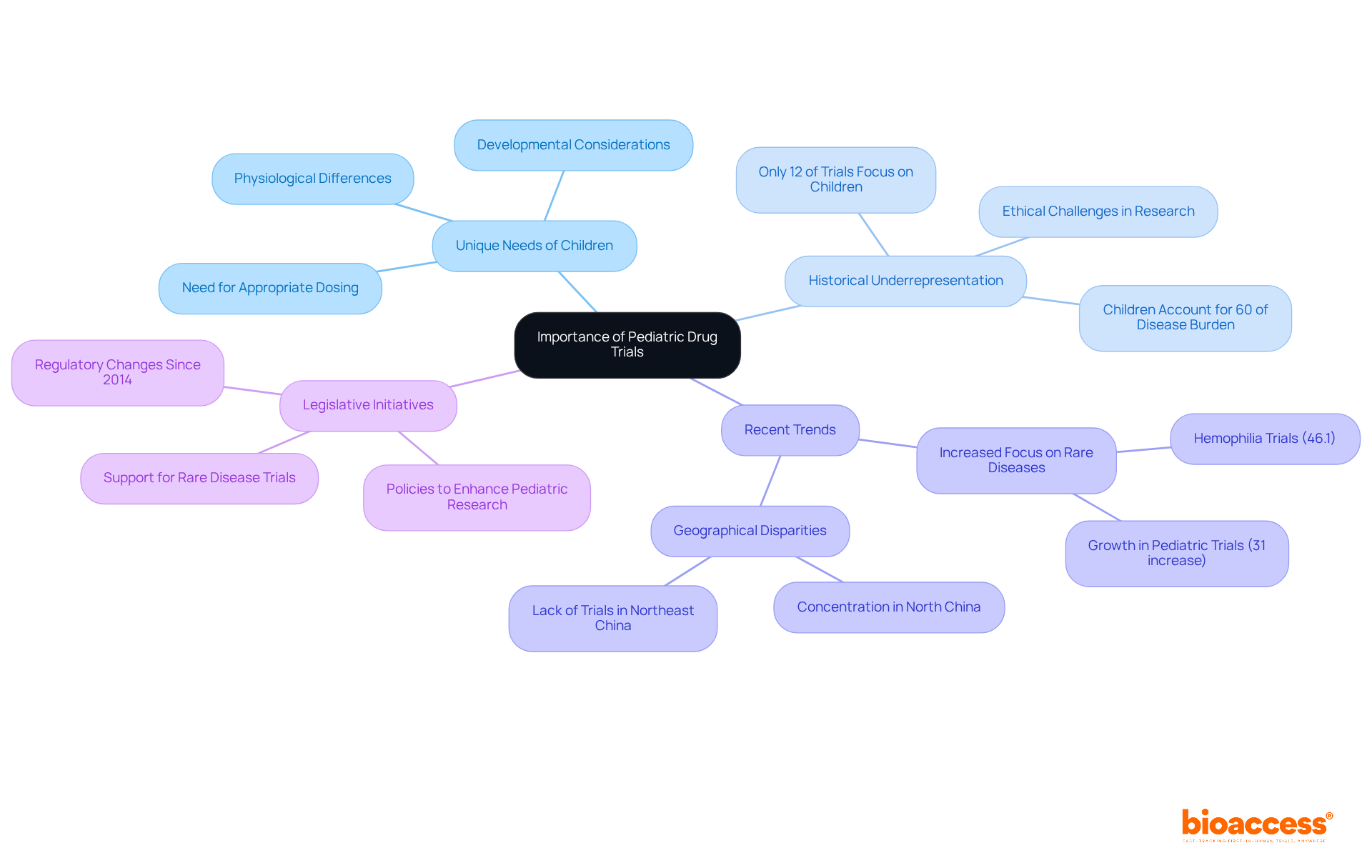
Historically, children have been significantly underrepresented in clinical studies, with a mere 12% of clinical medication assessments focusing on young patients, despite children accounting for nearly 60% of the disease burden for certain conditions. This substantial gap in knowledge regarding the safety and efficacy of numerous medications used in pediatric healthcare highlights the urgent need to prioritize studies involving children. As Dr. Dianne Murphy points out, innovative strategies are essential to guarantee long-term drug safety in this vulnerable population.
Recent research indicates that studies involving children have notably increased attention to rare conditions, such as hemophilia, which constitutes 46.1% of youth orphan disease studies. This trend further emphasizes the critical role of this research in improving medication safety and effectiveness for at-risk groups. Additionally, addressing geographical disparities in children's studies is imperative, as many regions still lack adequate representation in medical research.
Legislative initiatives aimed at enhancing research for children's medications are crucial for fostering a more inclusive and effective approach to pediatric drug trial authorization in Montenegro. By prioritizing these efforts, we can ensure that the unique needs of children are met in clinical research.
The regulatory framework for pediatric drug trial authorization in Montenegro is pivotal in ensuring ethical and effective clinical research for children's drug studies. Governed by essential laws and guidelines that align with EU standards, the Law on Medicines and the Law on Health Care delineate the requirements for conducting clinical studies. These laws emphasize the necessity for ethical approval and adherence to Good Clinical Practice (GCP), which are crucial for safeguarding young participants.
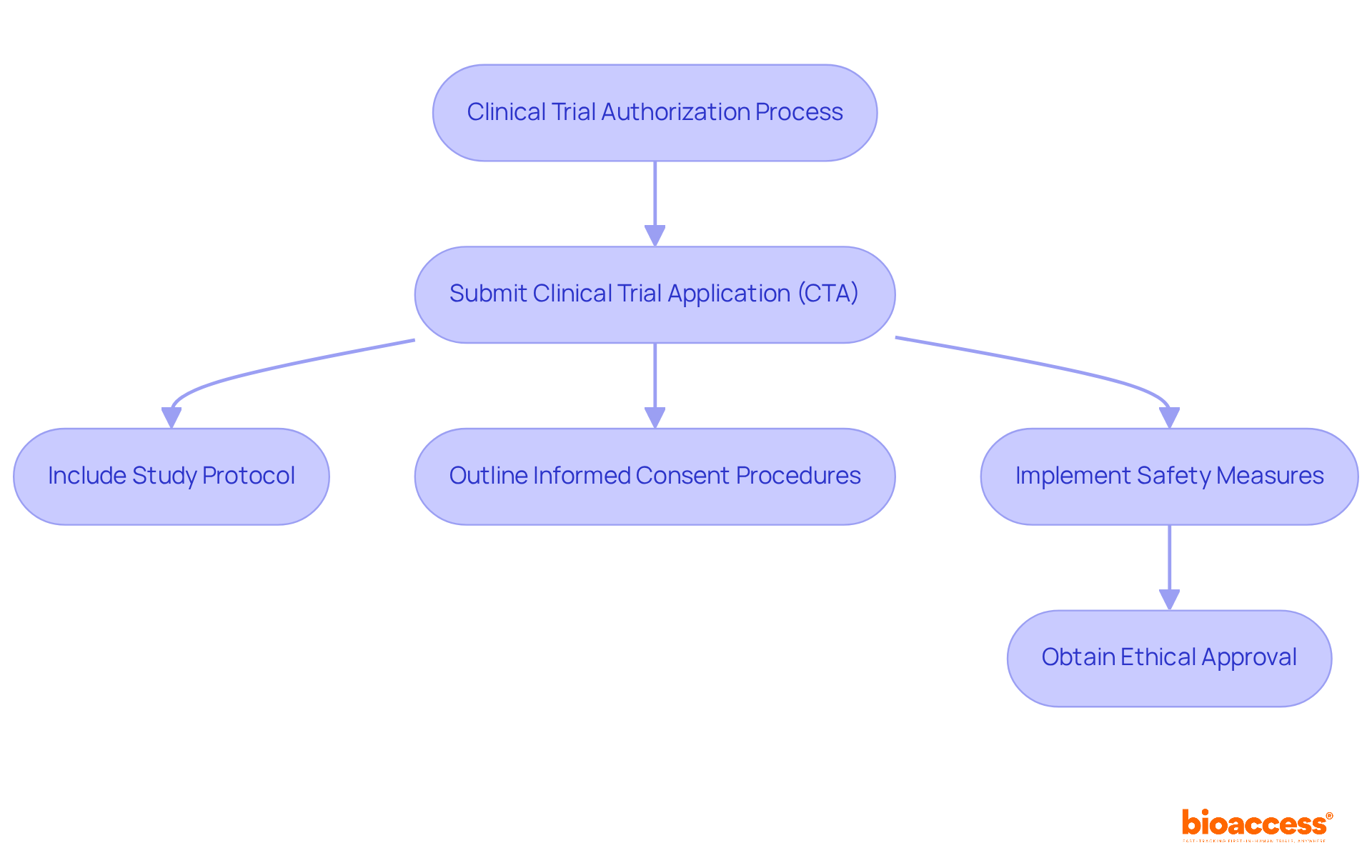
Researchers must submit a Clinical Trial Application (CTA) to the relevant authorities. This application must include:
Ethical considerations unique to child populations are paramount; obtaining assent from minors and consent from guardians is essential to prioritize the rights and welfare of young participants.
Adhering to these regulations is not just a matter of compliance; it is fundamental for the successful implementation of children's studies. Numerous examples of regulatory adherence throughout the EU demonstrate the effectiveness of these frameworks. Understanding these regulations is essential for researchers aiming to navigate the complexities of pediatric drug trial authorization in Montenegro effectively.
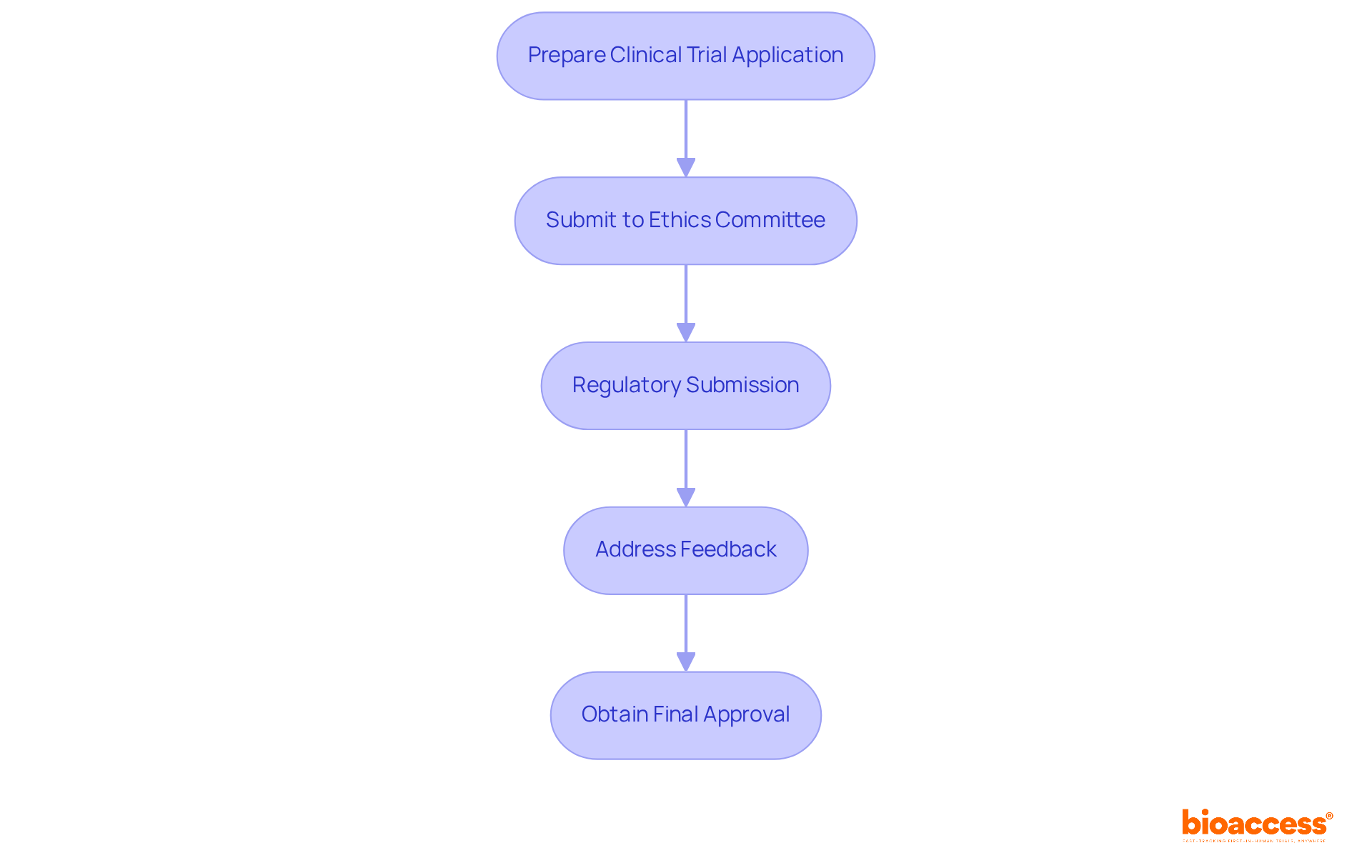
Navigating the pediatric drug trial authorization in Montenegro is crucial for ensuring compliance and safeguarding young participants. Researchers must pay careful attention to detail and adhere to regulatory standards. Here are the essential steps to follow:
Prepare the Clinical Trial Application (CTA): Begin by gathering a comprehensive study protocol, informed consent documents, and safety monitoring plans tailored for children. This preparation is foundational, as it sets the stage for the entire study.
Submit to the Ethics Committee: Securing approval from an ethics committee is vital. This committee assesses the ethical implications of the study, with a particular focus on the protection and welfare of child participants. As specialists emphasize, obtaining informed consent for child studies is more complex than for adults, requiring thoughtful communication of risks and benefits to both guardians and children.
Regulatory Submission: After receiving ethical approval, the next step is to submit the CTA to the Agency for Medicines and Medical Devices of Montenegro (CALIMS). This submission must adhere to all regulatory requirements to ensure compliance and facilitate a smooth review process.
Address Feedback: Researchers should be ready to respond promptly to any inquiries or requests for additional information from CALIMS. Effective communication can significantly expedite the approval timeline.
Obtain Final Approval: Once all requirements are met, researchers will receive final authorization to commence the study. Typically, the duration for pediatric drug trial authorization in Montenegro is about 4-6 weeks, which is notably efficient compared to many other regions.
By leveraging bioaccess's extensive research study management services-including feasibility assessments, site selection, compliance evaluations, import permits, and project oversight-researchers can streamline the process and minimize delays. This proactive approach helps overcome the regulatory challenges often faced in early-phase research studies.
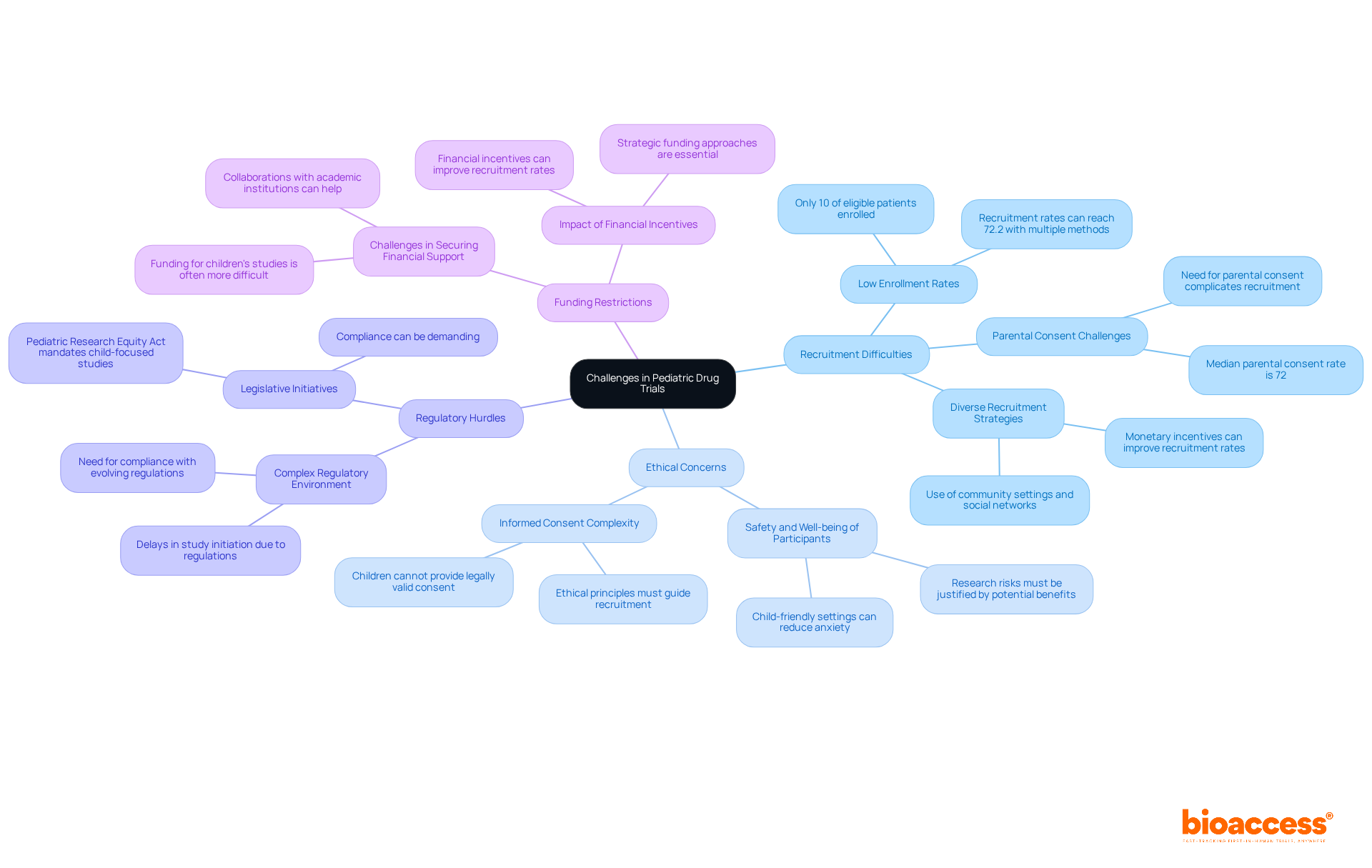
Carrying out child drug studies presents unique challenges that demand attention in clinical research. Recruitment Difficulties stand out as a significant hurdle. Finding eligible participants can be particularly challenging due to strict inclusion criteria and the necessity of obtaining parental consent. Engaging pediatricians and community organizations is essential for raising awareness and facilitating recruitment. Studies indicate that using multiple recruitment methods can significantly enhance participation rates, with a combined approach yielding recruitment rates as high as 72.2%. Additionally, employing various recruitment strategies resulted in a higher recruitment rate of 56.6% (95% CI: 24.5-86.0). Alarmingly, only 10% of eligible patients based on inclusion and exclusion criteria are enrolled in research studies, underscoring the considerable recruitment challenges encountered in trials involving children.
Ethical Concerns are paramount in this context. The safety and well-being of child participants must be the foremost priority. Researchers are required to navigate complex ethical considerations, ensuring transparent communication with parents and guardians about the risks and benefits involved. The principle of protection in pediatric research mandates that children should only be exposed to research risks when there is a prospect of direct benefit or minimal risk justified by the knowledge gained. Child-friendly settings and trained child-life specialists can also reduce anxiety and distress during clinical procedures, enhancing the overall experience for participants.
Regulatory Hurdles further complicate the landscape. Navigating the intricate regulatory environment can lead to delays in study initiation and execution. Staying informed about evolving regulations and maintaining open lines of communication with regulatory bodies are crucial strategies to mitigate these issues. Legislative initiatives, such as the Pediatric Research Equity Act, highlight the necessity for child-focused studies, yet compliance can be demanding.
Funding Restrictions pose another challenge. Obtaining financial support for children's studies frequently appears more challenging than for adult studies. Collaborating with academic institutions and leveraging grants specifically aimed at pediatric research can provide necessary financial support. Financial incentives have been shown to improve recruitment rates, highlighting the importance of strategic funding approaches.
By proactively addressing these challenges, researchers can significantly enhance the likelihood of successful trial outcomes.
The significance of pediatric drug trials is paramount, particularly in ensuring that children receive safe and effective medications tailored to their unique needs. The underrepresentation of children in clinical research poses serious risks, underscoring the urgent need for comprehensive studies that prioritize their health and well-being. By delving into the intricacies of pediatric drug trial authorization in Montenegro, researchers can contribute to a more equitable healthcare landscape for younger populations.
Key points discussed include:
Compliance with established guidelines - such as obtaining informed consent and ensuring the ethical treatment of child participants - is crucial for the success of these studies. Moreover, addressing recruitment difficulties and navigating regulatory hurdles are vital steps toward enhancing the participation and safety of children in clinical trials.
In conclusion, the call to action is clear: prioritizing pediatric drug trials is essential for advancing child health. Stakeholders must collaborate to overcome existing barriers and advocate for legislative initiatives that promote inclusive research practices. By doing so, Montenegro can ensure that its youngest citizens benefit from advancements in medical science, paving the way for a healthier future for all children.
Why are pediatric drug trials important?
Pediatric drug trials are essential for developing safe and effective treatments specifically tailored for children, as they metabolize medications differently than adults.
How do children differ from adults in terms of medication metabolism?
Children have unique physiological and developmental characteristics that lead to different metabolism of medications compared to adults, necessitating studies specifically for this age group.
What is the current representation of children in clinical studies?
Historically, children have been underrepresented in clinical studies, with only 12% of clinical medication assessments focusing on young patients, despite children accounting for nearly 60% of the disease burden for certain conditions.
What are the implications of the lack of pediatric drug trials?
The lack of pediatric drug trials results in substantial gaps in knowledge regarding the safety and efficacy of many medications used in pediatric healthcare, highlighting the urgent need for more studies involving children.
What recent trends have been observed in pediatric drug studies?
Recent research indicates an increase in studies focusing on rare conditions, such as hemophilia, which makes up 46.1% of youth orphan disease studies, emphasizing the importance of pediatric research for at-risk groups.
Why is addressing geographical disparities in children's studies important?
Addressing geographical disparities is crucial because many regions still lack adequate representation in medical research, which can affect the generalizability and safety of pediatric treatments.
What role do legislative initiatives play in pediatric drug trials?
Legislative initiatives are vital for enhancing research on children's medications and fostering a more inclusive and effective approach to pediatric drug trial authorization, ensuring that children's unique needs are met in clinical research.