


Phase 1b trials are pivotal in drug development, as they evaluate the safety and effectiveness of new treatments across a broader patient population. These trials often yield significant insights regarding optimal dosing and potential side effects. Emphasizing the necessity of robust trial design, the article addresses challenges in recruitment and compliance, alongside the critical need to adhere to regulatory and ethical standards. Such considerations are vital for ensuring the integrity and success of these trials.
Phase 1b trials are pivotal in the drug development process, serving as a critical link between initial safety assessments and comprehensive efficacy evaluations. These studies not only yield vital insights into a drug's potential but also encounter considerable challenges, particularly in recruitment and compliance. As clinical research becomes increasingly complex, researchers must navigate these obstacles while upholding stringent regulatory and ethical standards. This article explores the intricacies of Phase 1b trials, examining their design, the hurdles they face, and the innovative strategies that can bolster their success.
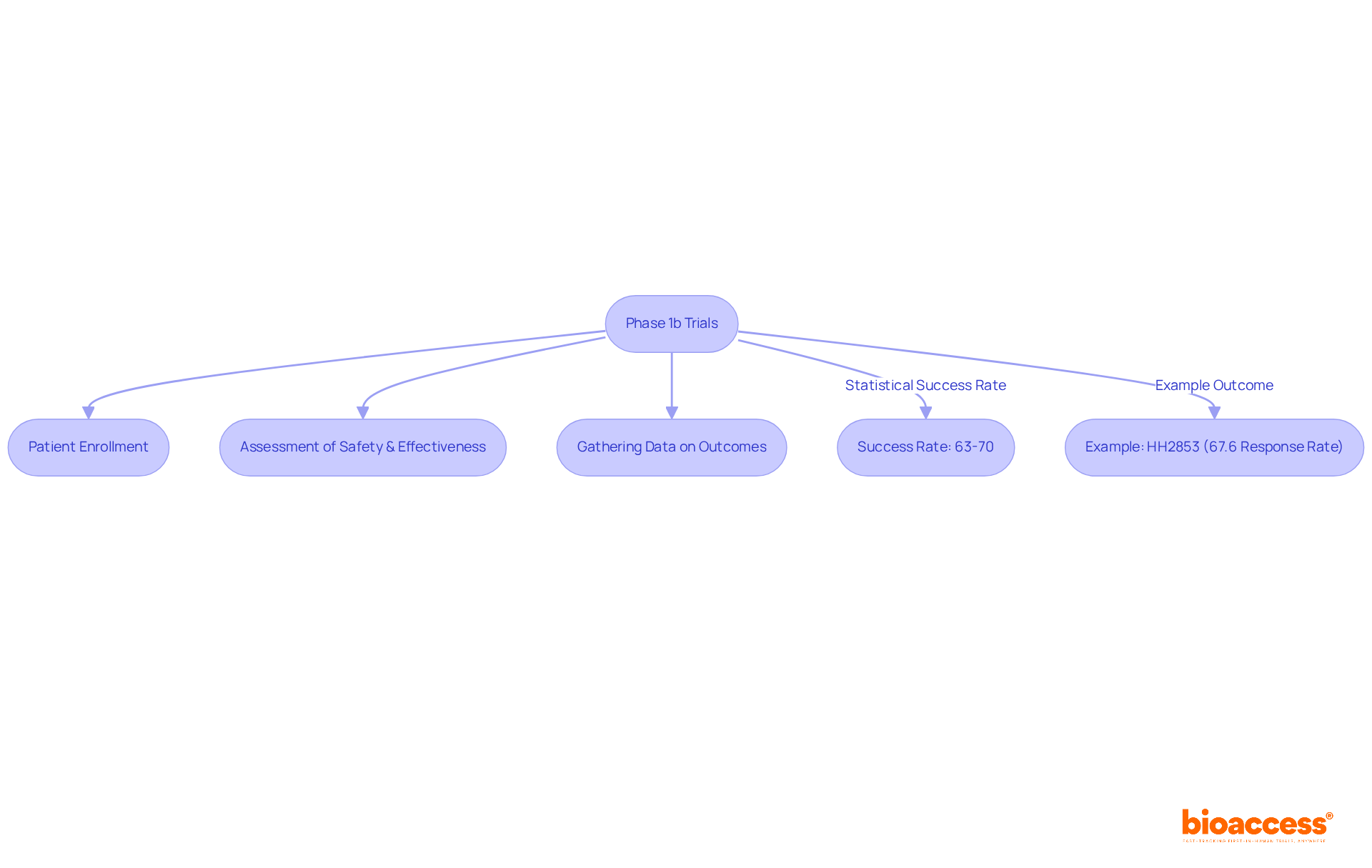
Phase 1b studies represent a pivotal stage in the development of new medications and treatments, focusing on the assessment of safety and effectiveness within a broader patient population compared to Phase 1a studies. These studies typically involve individuals diagnosed with the targeted condition, aiming to gather initial insights regarding the drug's effectiveness, optimal dosing, and potential side effects. Notably, approximately 63%-70% of medications successfully navigate through Stage 1 clinical evaluations, underscoring the critical nature of this phase in the overall drug development continuum.
For example, the phase 1b study of HH2853 in patients with relapsed/refractory peripheral T-cell lymphoma demonstrated an overall response rate of 67.6%, illustrating the substantial impact that phase 1b studies can have on expanding treatment options. As the landscape of drug development evolves, the relevance of phase 1b studies continues to grow, especially considering the increasing complexity of therapies and the demand for robust data to guide subsequent phases.
By harnessing bioaccess®'s capabilities, clinical researchers can enroll treatment-naive cohorts in cardiology or neurology 50% faster than traditional Western sites, achieving savings of $25K per patient with FDA-ready data—effectively eliminating rework and delays. This enhanced efficiency not only deepens the understanding of a drug's therapeutic potential but also plays a crucial role in shaping the future of drug development, ultimately leading to improved patient outcomes.
Designing a study in phase 1b necessitates meticulous attention to methodologies and protocols, ensuring robust data collection and analysis. Randomized controlled studies (RCTs) serve as a cornerstone of this design, where participants are randomly allocated to receive either the experimental treatment or a placebo. This approach effectively minimizes bias and facilitates a clearer comparison of outcomes.
Moreover, adaptive study designs are increasingly preferred, allowing for adjustments based on interim outcomes, thereby enhancing both efficiency and ethical considerations. Key components of study design include:
By carefully organizing these elements, researchers can strengthen the study's framework, producing significant outcomes that guide subsequent stages of development.
Bioaccess® accelerates patient enrollment for cardiology and neurology groups by 50% compared to Western locations and achieves regulatory approval in just 6-8 weeks, significantly streamlining the research process. Furthermore, bioaccess® offers extensive clinical study management services, including:
This ensures a comprehensive approach to study execution. The conventional 3 + 3 design remains an essential method in Stage 1 studies, providing a fundamental strategy for dose escalation and safety evaluation.

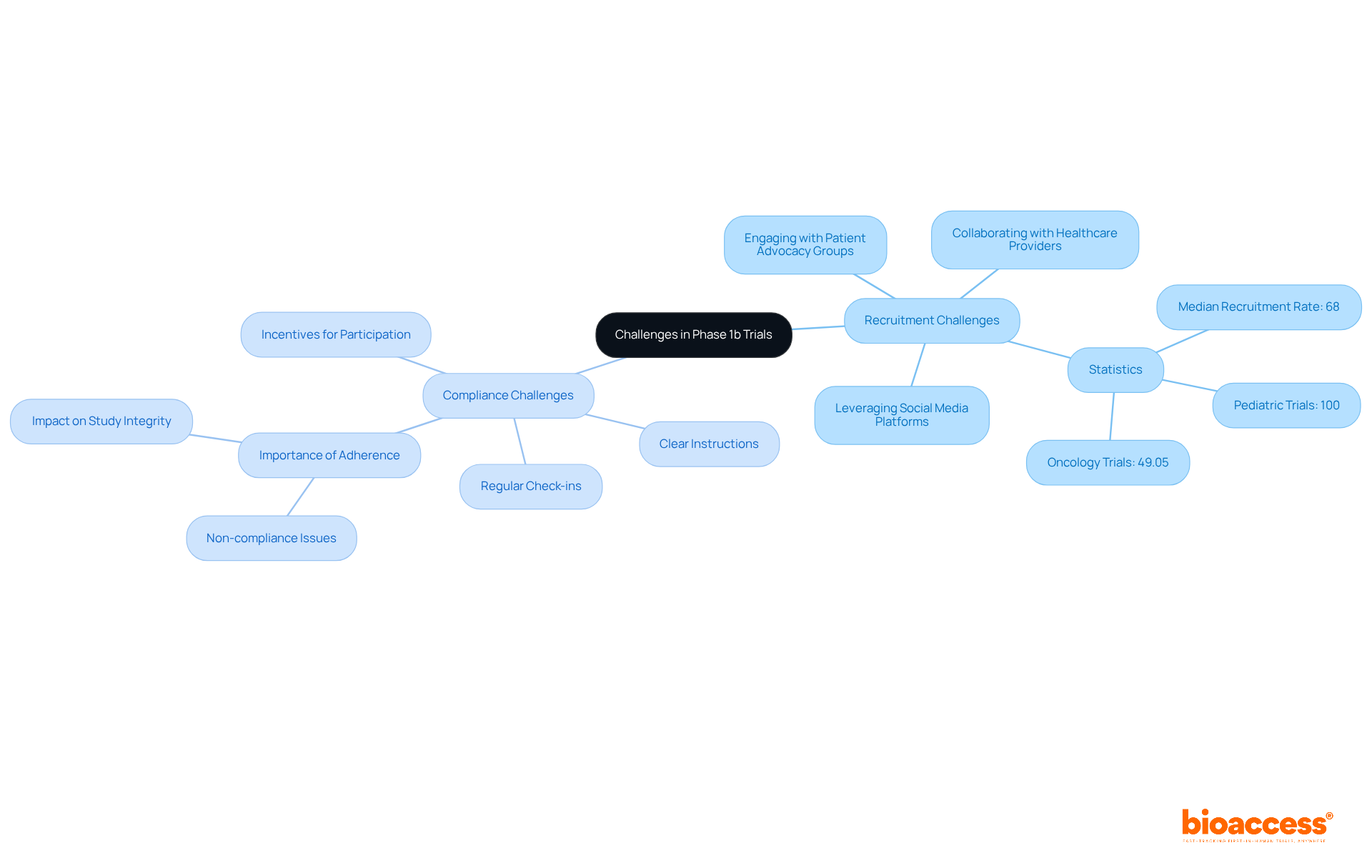
Recruitment and compliance present significant challenges in studies during Phase 1b. The specific eligibility criteria often limit the pool of potential participants, rendering recruitment particularly arduous. To bolster recruitment efforts, strategies such as:
can effectively raise awareness about the study. Statistics reveal that merely 36.32% of early-phase studies successfully meet their planned recruitment goals, highlighting the necessity for innovative approaches.
Adherence—referring to individuals following the study protocol, including medication schedules and follow-up appointments—is equally vital. Non-compliance can skew results and compromise the integrity of the study. To address compliance issues, researchers can implement:
Notably, pediatric studies have shown a median recruitment rate of 100%, indicating that tailored communication and support can significantly enhance involvement. By proactively tackling recruitment and compliance challenges, researchers can improve the likelihood of successful study completion and uphold data integrity.
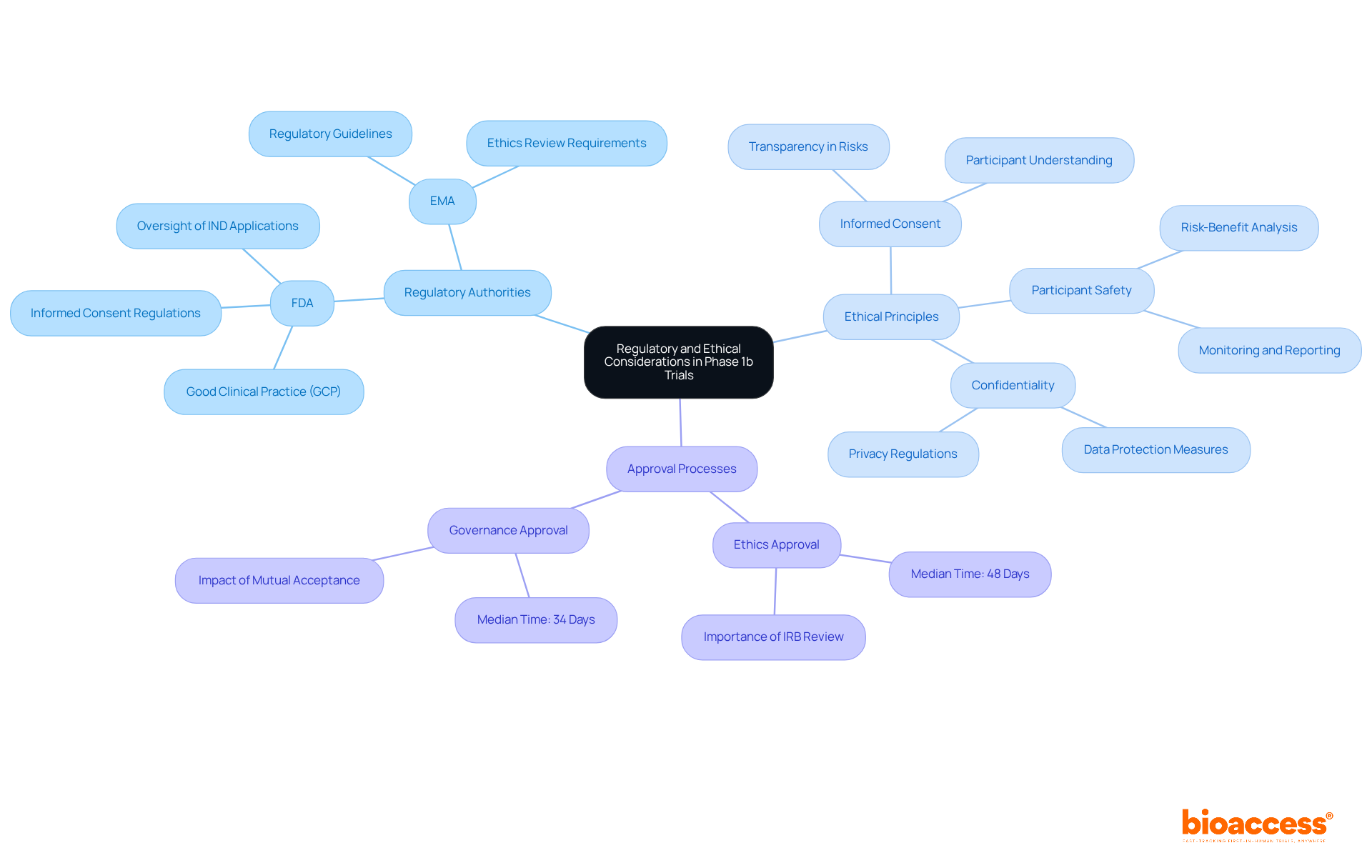
Studies in phase 1b are governed by stringent guidelines and ethical principles designed to protect subjects and uphold the integrity of research. Regulatory authorities, such as the FDA in the United States and the EMA in Europe, mandate strict adherence to Good Clinical Practice (GCP) guidelines. This includes:
A thorough review by an Institutional Review Board (IRB) or Ethics Committee (EC) is crucial for evaluating the study's risk-benefit ratio. Notably, the median duration for ethics approval in research trials is approximately 48 days, highlighting the importance of comprehensive review processes. Researchers are obligated to transparently communicate potential risks and ensure that participants are fully informed before providing consent. By prioritizing regulatory compliance and ethical standards, researchers not only protect subjects but also cultivate trust and integrity within the research environment.
Moreover, the adoption of streamlined ethics review processes has demonstrated potential in enhancing efficiency, with studies showing that mutual acceptance of ethics approvals can significantly decrease overall site activation times. This commitment to ethical standards is vital for advancing medical research while safeguarding the rights and welfare of all involved.
At bioaccess®, we specialize in comprehensive trial management services, encompassing:
Our expertise ensures meticulous attention to regulatory and ethical considerations, facilitating expedited clinical studies for medical devices while protecting the rights and welfare of participants.
Phase 1b trials are pivotal in the drug development process, concentrating on the assessment of safety and efficacy of new treatments within a broader patient population. These studies build upon the foundational insights gained from Phase 1a trials, yielding essential data that influences subsequent phases of clinical research. The importance of these trials is emphasized by their capacity to inform treatment options and enhance patient outcomes, establishing them as a vital element of medical advancement.
This article has explored key aspects of Phase 1b trials, including:
The implementation of randomized controlled trials and adaptive study designs bolsters the reliability of the data collected, while innovative strategies for recruitment and adherence can significantly influence the success of these studies. Moreover, a steadfast commitment to regulatory compliance and ethical standards safeguards participant protection and maintains the integrity of the research.
As the landscape of drug development evolves, the significance of Phase 1b trials remains paramount. They not only serve as a gateway for new therapies but also reflect the ongoing dedication to advancing medical science responsibly. By embracing innovative methodologies and addressing the inherent challenges of these trials, researchers can contribute to the future of healthcare, ultimately leading to safer and more effective treatments for patients. Engaging with the latest trends and strategies in Phase 1b trials is crucial for all stakeholders involved in clinical research, paving the way for breakthroughs that can transform lives.
What are Phase 1b trials?
Phase 1b trials are a stage in the development of new medications that assess safety and effectiveness within a broader patient population, focusing on individuals diagnosed with the targeted condition.
What is the purpose of Phase 1b studies?
The purpose of Phase 1b studies is to gather initial insights regarding a drug's effectiveness, optimal dosing, and potential side effects.
How successful are medications in Phase 1 clinical evaluations?
Approximately 63%-70% of medications successfully navigate through Stage 1 clinical evaluations.
Can you provide an example of a Phase 1b study?
An example is the phase 1b study of HH2853 in patients with relapsed/refractory peripheral T-cell lymphoma, which demonstrated an overall response rate of 67.6%.
Why are Phase 1b studies becoming more relevant?
Phase 1b studies are becoming more relevant due to the increasing complexity of therapies and the demand for robust data to guide subsequent phases of drug development.
How does bioaccess® enhance the efficiency of clinical research?
Bioaccess® enables clinical researchers to enroll treatment-naive cohorts in cardiology or neurology 50% faster than traditional Western sites, achieving savings of $25K per patient with FDA-ready data.
What impact do Phase 1b studies have on drug development?
Phase 1b studies deepen the understanding of a drug's therapeutic potential and play a crucial role in shaping the future of drug development, ultimately leading to improved patient outcomes.