


Clinical trials serve as the backbone of medical advancement, yet the safety of participants remains paramount. The meticulous process of safety reporting is essential, not only for regulatory compliance but also for safeguarding the health of those involved in these studies. Alarming statistics reveal that a significant number of serious adverse events go unreported, highlighting the critical need for clarity and adherence to safety protocols.
How can researchers effectively navigate the complexities of safety reporting under Halmed's guidelines while maintaining the integrity of their trials?
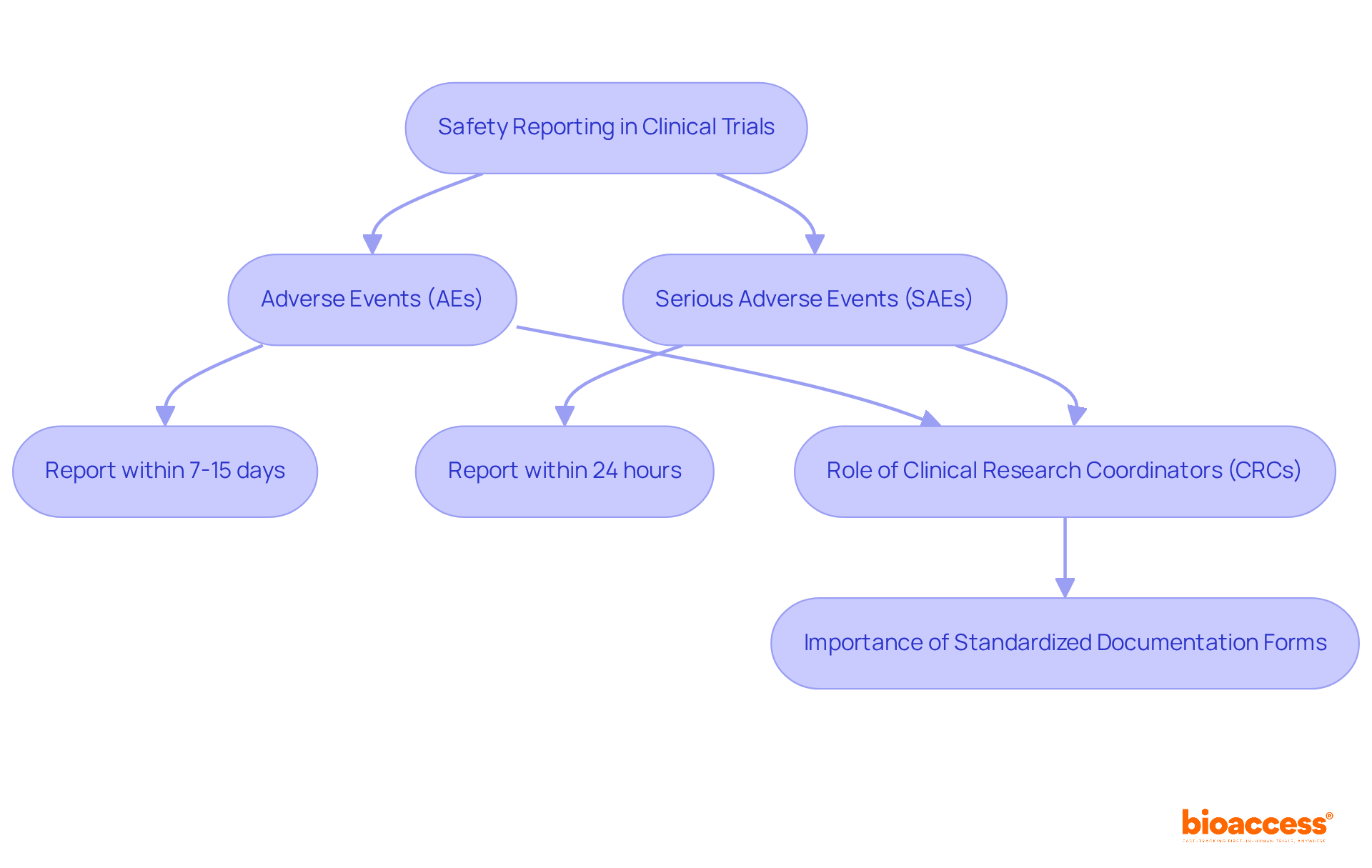
Safety documentation in clinical trials is a systematic process that involves the collection and analysis of data regarding adverse events (AEs) that occur during the study. This process is crucial for recognizing potential risks linked to investigational products and ensuring the well-being of participants.
Adverse Events (AEs) are any undesirable experiences associated with the use of a medical product, ranging from mild symptoms to severe conditions. On the other hand, Serious Adverse Events (SAEs) are characterized as AEs that lead to significant outcomes like death, hospitalization, or severe disability. SAEs necessitate immediate attention and notification. Regulatory authorities require that serious adverse occurrences must be reported within 24 hours for life-threatening situations, while other AEs should be reported within 7 to 15 days, depending on their severity and the trial phase. Timely updates are crucial to mitigate risks and ensure participant safety.
The significance of precise adverse occurrence reporting cannot be overstated. In 2025, it was discovered that a shocking percentage of severe negative occurrences were under-reported in clinical studies, with research indicating that up to 92% of serious adverse incidents were not sufficiently recorded. This under-reporting can lead to significant gaps in safety data, potentially compromising patient health and the integrity of the trial.
Instances of negative occurrence documentation methods emphasize the necessity for thorough record-keeping. Clinical Research Coordinators (CRCs) play a vital role in maintaining accurate records of AEs, ensuring that all necessary details - including patient information, event descriptions, and the relationship to the study drug - are communicated effectively to sponsors and regulatory authorities. Furthermore, the implementation of standardized documentation forms, such as the FDA's MedWatch, is essential for compliance with regulatory guidelines.
In conclusion, comprehending and following the safety reporting protocol for trials under halmed is vital for preserving the integrity of clinical studies and ensuring participant well-being. Efficient adverse occurrence documentation not only meets ethical responsibilities but also boosts the trustworthiness of clinical research.
Halmed, the Croatian Agency for Medicinal Products and Medical Devices, has established a safety reporting protocol for trials under halmed, which is crucial for guaranteeing participant welfare and ensuring regulatory adherence. These requirements are not just guidelines; they are essential for maintaining the integrity of clinical research and ensuring the safety reporting protocol for trials under halmed, which safeguards the well-being of participants.
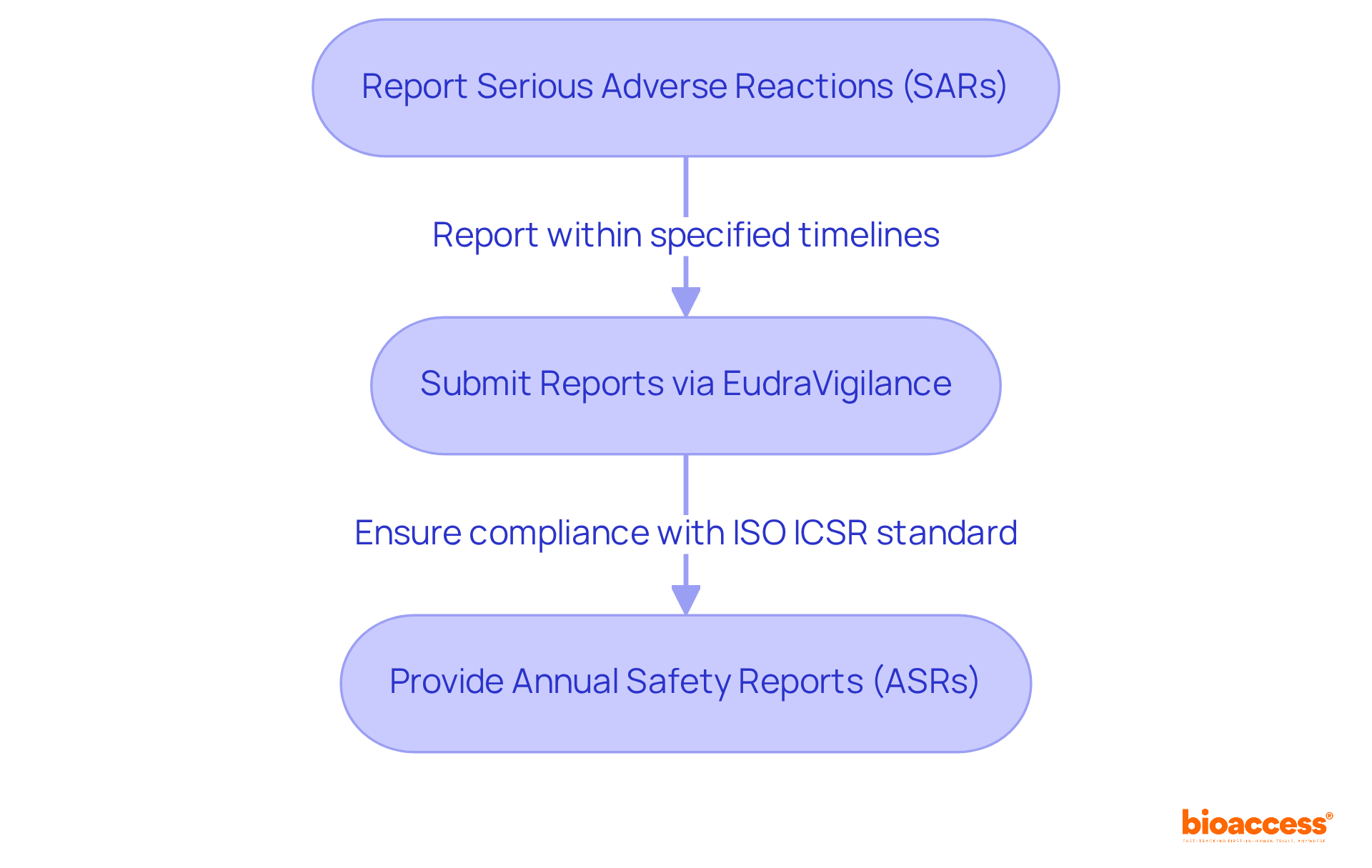
Submission of Safety Information: All serious adverse reactions (SARs) must be reported to Halmed within specified timelines. The safety reporting protocol for trials under halmed emphasizes the importance of timely reporting. Studies indicate that complete reporting of serious adverse events is significantly higher in structured registries like ClinicalTrials.gov, reaching 100% compared to 79.2% in publications. This statistic underscores the importance of adhering to the safety reporting protocol for trials under halmed in order to meet these reporting timelines.
Use of EudraVigilance: Reports should be submitted through the EudraVigilance system, a centralized database maintained by the European Medicines Agency (EMA) since 2001. This system enhances the oversight of drug integrity across Europe, facilitating the electronic exchange of individual case reports (ICSRs) among stakeholders. The compulsory implementation of the ISO ICSR standard, effective from June 30, 2022, further simplifies this process, ensuring uniformity and dependability in data submissions.
Annual Safety Reports (ASRs): Sponsors must provide ASRs at consistent intervals, summarizing data and risk evaluations. This practice is essential, as it allows for continuous assessment of results and compliance with the safety reporting protocol for trials under Halmed regulations. Notably, adherence rates for risk documentation in clinical studies have improved, with research indicating that almost 58% of studies convey risk outcomes in ClinicalTrials.gov. This highlights the significance of employing such registries for thorough risk information.
Following these requirements is vital for upholding compliance and ensuring participant well-being during the safety reporting protocol for trials under halmed. Incomplete reporting can lead to serious regulatory consequences and jeopardize patient welfare. Therefore, it is imperative for all stakeholders to prioritize these guidelines.
To effectively submit safety information to Halmed, it’s essential to follow these detailed steps:
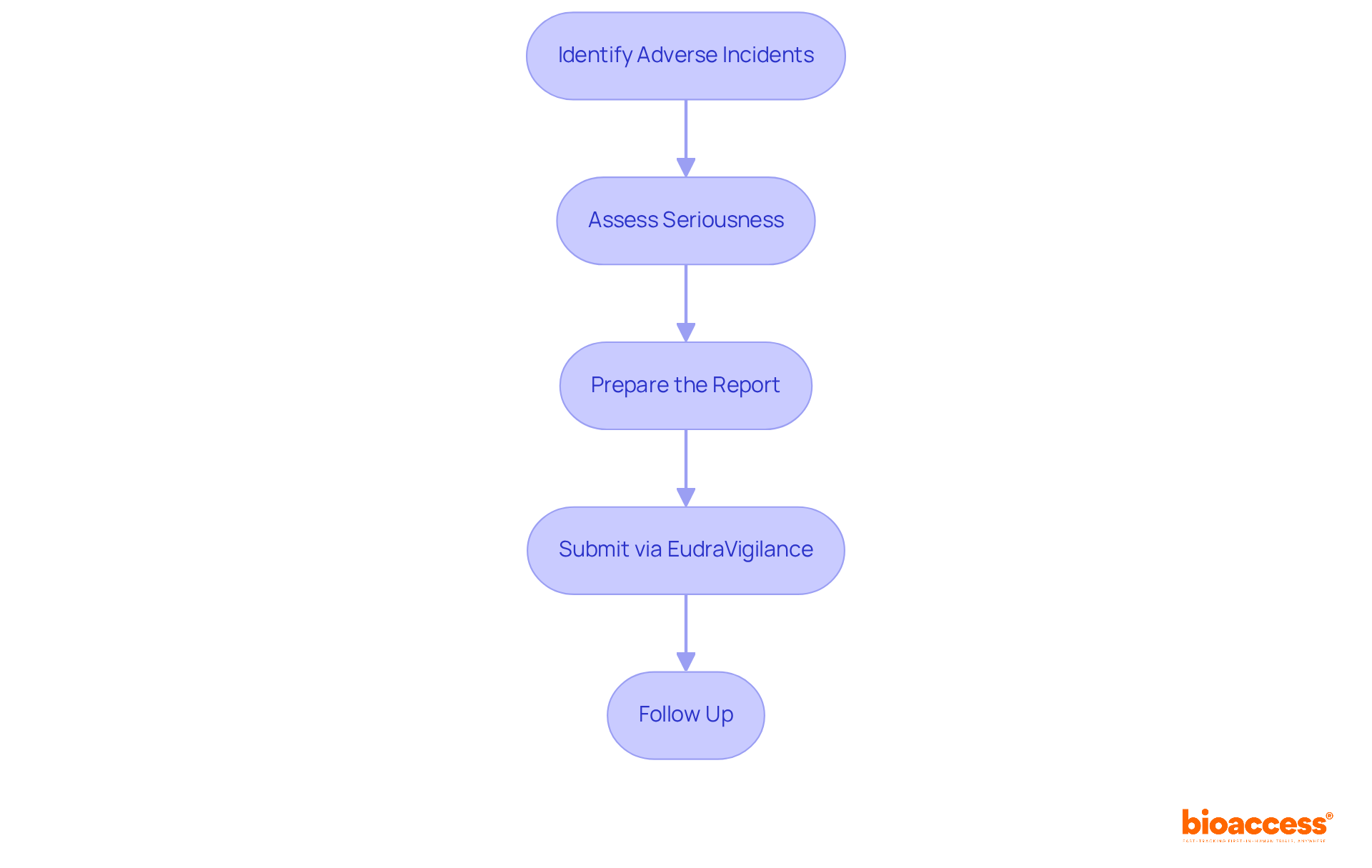
Identify Adverse Incidents: Vigilantly monitor and document any adverse occurrences (AEs) that arise during the trial. AEs can range from mild symptoms to severe conditions, and recognizing them early is crucial for participant safety.
Assess Seriousness: Evaluate whether the occurrence meets the criteria for seriousness. Serious adverse occurrences (SAOs) include outcomes such as death, life-threatening situations, hospitalization, or disability. Understanding the distinction between AEs and SAEs is vital for proper reporting and management.
Prepare the Report: Compile comprehensive information for the report, including patient details, the nature of the occurrence, its severity, and the outcome. Documentation should be thorough, as it supports regulatory compliance and participant safety. Reports must include details such as the date of onset, resolution, and any actions taken in response to the occurrence.
Submit via EudraVigilance: Access the EudraVigilance system to complete the submission form. Ensure that all required fields are filled out accurately to facilitate a smooth review process. Prompt documentation is crucial; significant negative occurrences must be communicated within 24 hours to prevent regulatory penalties, as required by the FDA and EMA.
Follow Up: After submission, actively monitor for any feedback or requests for additional information from Halmed. Continuous communication is key to addressing any concerns that may arise during the review process.
By adhering to the safety reporting protocol for trials under Halmed, researchers can guarantee the prompt and precise communication of risk information, which is essential for preserving participant well-being and regulatory adherence in clinical studies. Consistent training on adverse event documentation guidelines can further reduce mistakes and delays, improving the overall integrity of the study.
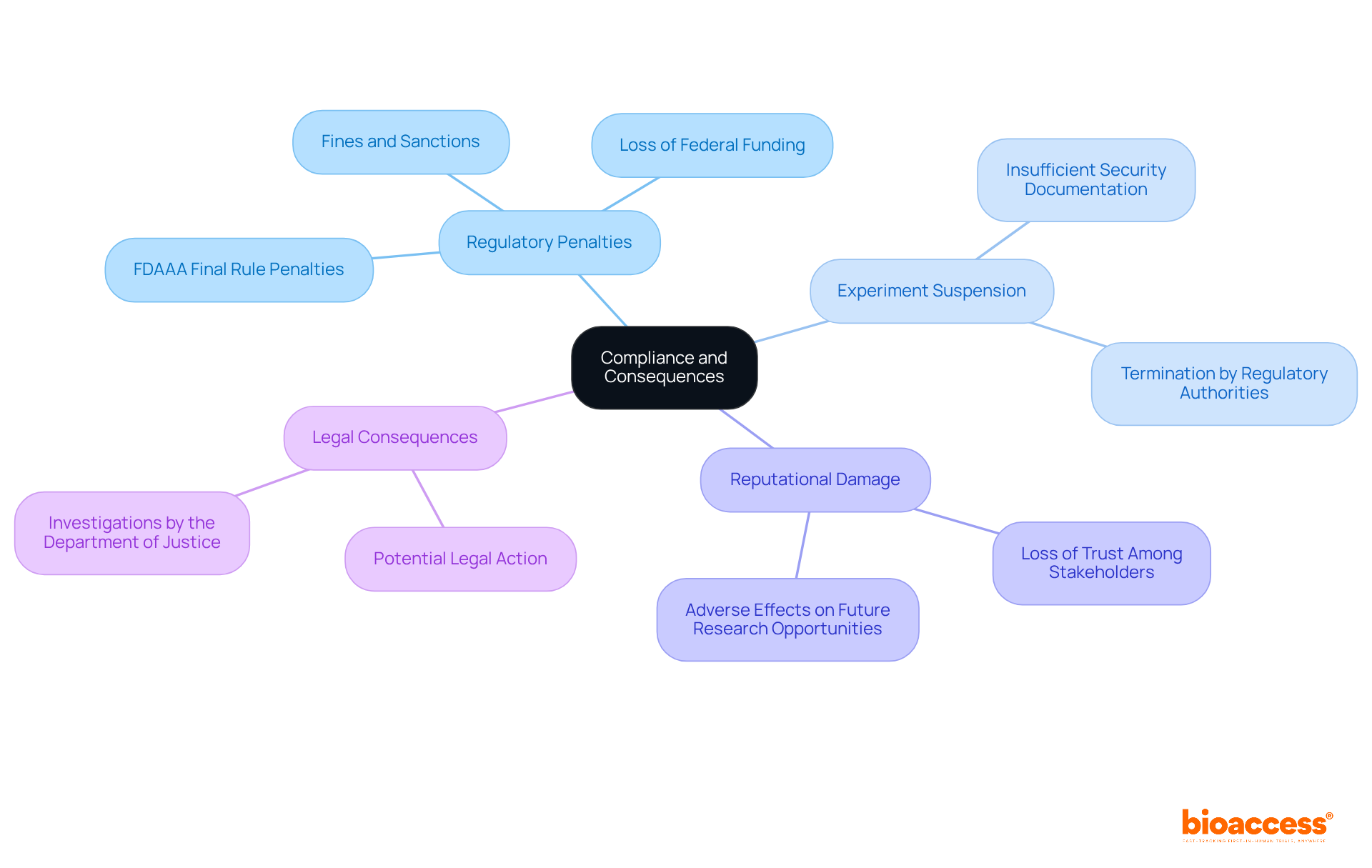
It is crucial for the success of clinical studies to adhere to the safety reporting protocol for trials under halmed. Non-compliance can lead to significant consequences, including:
The impact of non-compliance on clinical study success rates is profound. Research indicates that studies with higher compliance rates are more likely to achieve successful outcomes and prompt reporting. For instance, industry-sponsored studies reported a compliance rate of 50%, compared to only 31% for government-sponsored research, highlighting the importance of adherence to safety protocols.
To mitigate these risks, it is crucial for researchers to prioritize compliance with the safety reporting protocol for trials under halmed and maintain thorough documentation throughout the trial process. Establishing robust training and procedures can ease the compliance burden and enhance the integrity of clinical research.
Understanding and adhering to the safety reporting protocol for trials under Halmed is essential for ensuring participant safety and maintaining the integrity of clinical research. This protocol not only protects the well-being of individuals involved in clinical trials but also upholds the ethical standards required in the medical field.
The article highlights the critical components of safety reporting, including:
It underscores the role of Clinical Research Coordinators in meticulous documentation and emphasizes the mandatory use of systems like EudraVigilance for accurate submissions. Furthermore, the potential consequences of non-compliance, such as regulatory penalties and reputational damage, serve as a stark reminder of the stakes involved in clinical research.
In light of these insights, it is imperative for all stakeholders involved in clinical trials to prioritize compliance with safety reporting protocols. By doing so, researchers not only safeguard participant health but also contribute to the reliability and credibility of the clinical research landscape. Upholding these standards is not just a regulatory obligation; it is a commitment to ethical research practices that ultimately benefit society as a whole.
What is the purpose of safety documentation in clinical trials?
Safety documentation in clinical trials is a systematic process aimed at collecting and analyzing data regarding adverse events (AEs) to recognize potential risks linked to investigational products and ensure the well-being of participants.
What are Adverse Events (AEs) and Serious Adverse Events (SAEs)?
Adverse Events (AEs) are any undesirable experiences associated with the use of a medical product, which can range from mild symptoms to severe conditions. Serious Adverse Events (SAEs) are AEs that lead to significant outcomes such as death, hospitalization, or severe disability.
What are the reporting timelines for Serious Adverse Events (SAEs) and other AEs?
Serious Adverse Events must be reported within 24 hours for life-threatening situations, while other AEs should be reported within 7 to 15 days, depending on their severity and the trial phase.
Why is accurate reporting of adverse events important in clinical trials?
Accurate reporting of adverse events is crucial to mitigate risks, ensure participant safety, and maintain the integrity of clinical trials. Under-reporting can lead to significant gaps in safety data, potentially compromising patient health.
What role do Clinical Research Coordinators (CRCs) play in safety reporting?
Clinical Research Coordinators (CRCs) are responsible for maintaining accurate records of AEs, ensuring that all necessary details, including patient information and event descriptions, are effectively communicated to sponsors and regulatory authorities.
What standardized documentation forms are used for compliance in safety reporting?
Standardized documentation forms, such as the FDA's MedWatch, are essential for compliance with regulatory guidelines in the reporting of adverse events.
What is the overall significance of understanding safety reporting protocols in clinical trials?
Understanding and following safety reporting protocols is vital for preserving the integrity of clinical studies and ensuring participant well-being, as it meets ethical responsibilities and enhances the trustworthiness of clinical research.