


Simple randomisation in clinical trials serves as a fundamental method that allocates participants to treatment groups purely by chance. This approach guarantees that each individual possesses an equal opportunity to be assigned to any category, thereby minimizing bias and enhancing the study's validity. Such a technique is vital for maintaining internal validity, as it allows researchers to directly attribute differences in outcomes to the treatment. Consequently, this supports the integrity and reliability of clinical research findings, underscoring the importance of rigorous methodologies in advancing medical knowledge.
In the realm of clinical trials, the integrity of study results is fundamentally linked to the methods employed for participant assignment to treatment groups. Simple randomisation emerges as a pivotal technique, guaranteeing that each participant possesses an equal opportunity of being allocated to any group. This approach minimizes bias and significantly enhances the validity of findings. This article explores the essential steps for mastering simple randomisation, providing researchers with a clear roadmap to effectively implement this critical process. However, when the simplicity of randomisation encounters common pitfalls, what strategies can researchers employ to navigate these challenges and uphold the reliability of their trials?
In clinical studies, simple randomisation is a crucial technique used to allocate individuals to various treatment categories solely by chance. This method ensures that every individual has an equal opportunity to be assigned to any category, typically achieved through simple randomisation or straightforward methods, such as flipping a coin.
For example, in an experiment with two sections—one for treatment and one for control—a coin toss can decisively determine which section an individual will join. This straightforward approach, which includes simple randomisation, not only minimizes bias but also establishes a fundamental methodology in clinical research, underscoring its importance in ensuring the integrity of study results.
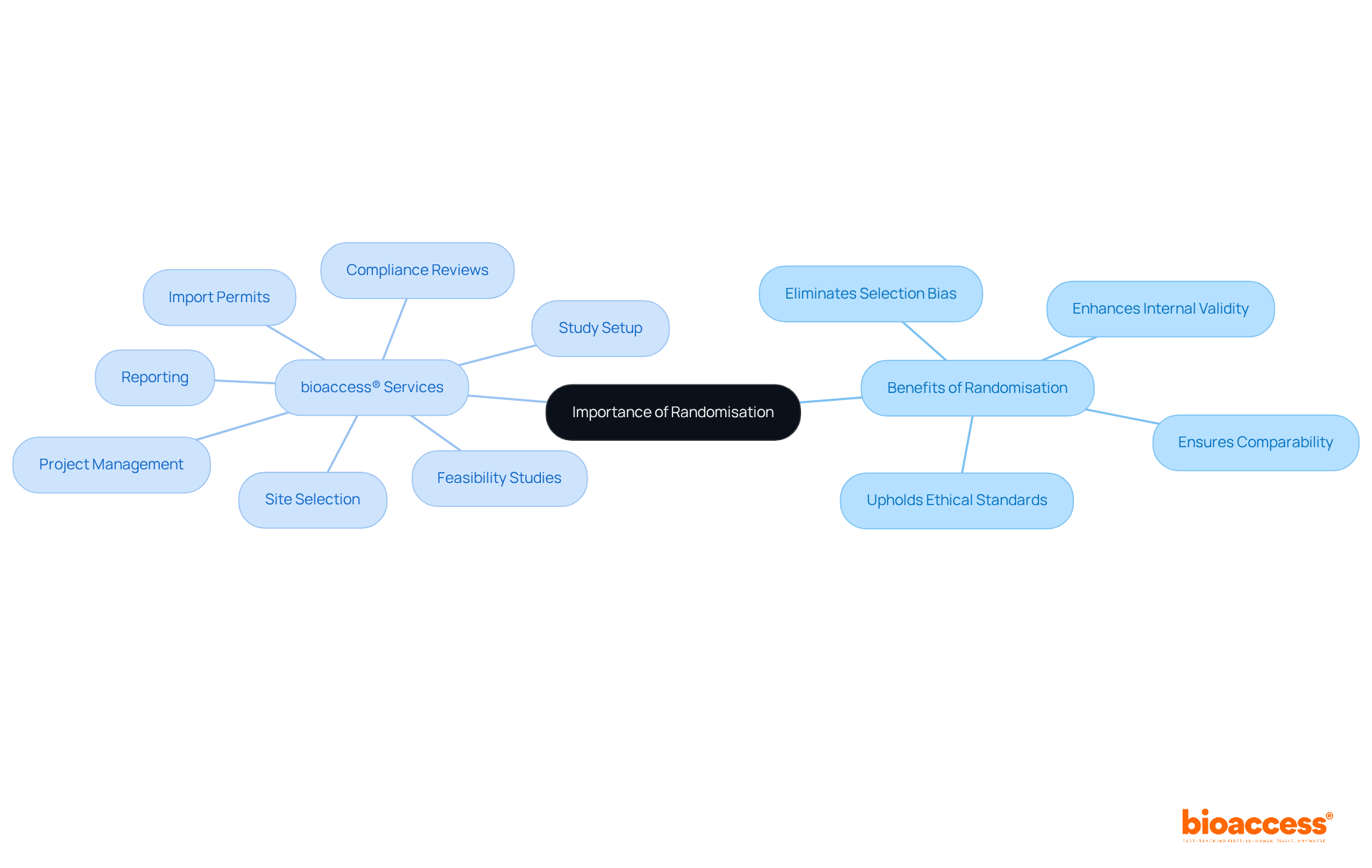
Simple randomisation is a cornerstone of clinical studies, effectively eliminating selection bias and ensuring comparability between treatment and control groups. By employing simple randomisation to assign subjects, researchers can confidently attribute differences in outcomes to the treatment itself, rather than to pre-existing disparities among subjects. This method, which incorporates simple randomisation, significantly enhances the internal validity of the trial, making the findings more reliable and applicable to the broader population.
Moreover, the allocation process upholds ethical standards in research by utilizing simple randomisation to ensure that all individuals have an equal chance to receive potentially advantageous treatments. For instance, in a study evaluating the effect of a mobile health initiative on sexual and reproductive health, simple randomisation was used to assign participants, ensuring even distribution across intervention and control groups, thereby reducing bias and improving the reliability of the findings.
At bioaccess®, with over 20 years of experience in Medtech, our extensive clinical study management services in Latin America encompass:
This rigorous application of simple randomisation not only strengthens the integrity of clinical studies but also contributes to more accurate and generalizable findings, particularly within the context of our specialized services in Early-Feasibility, First-In-Human, Pilot, Pivotal, and Post-Market Follow-Up Research.
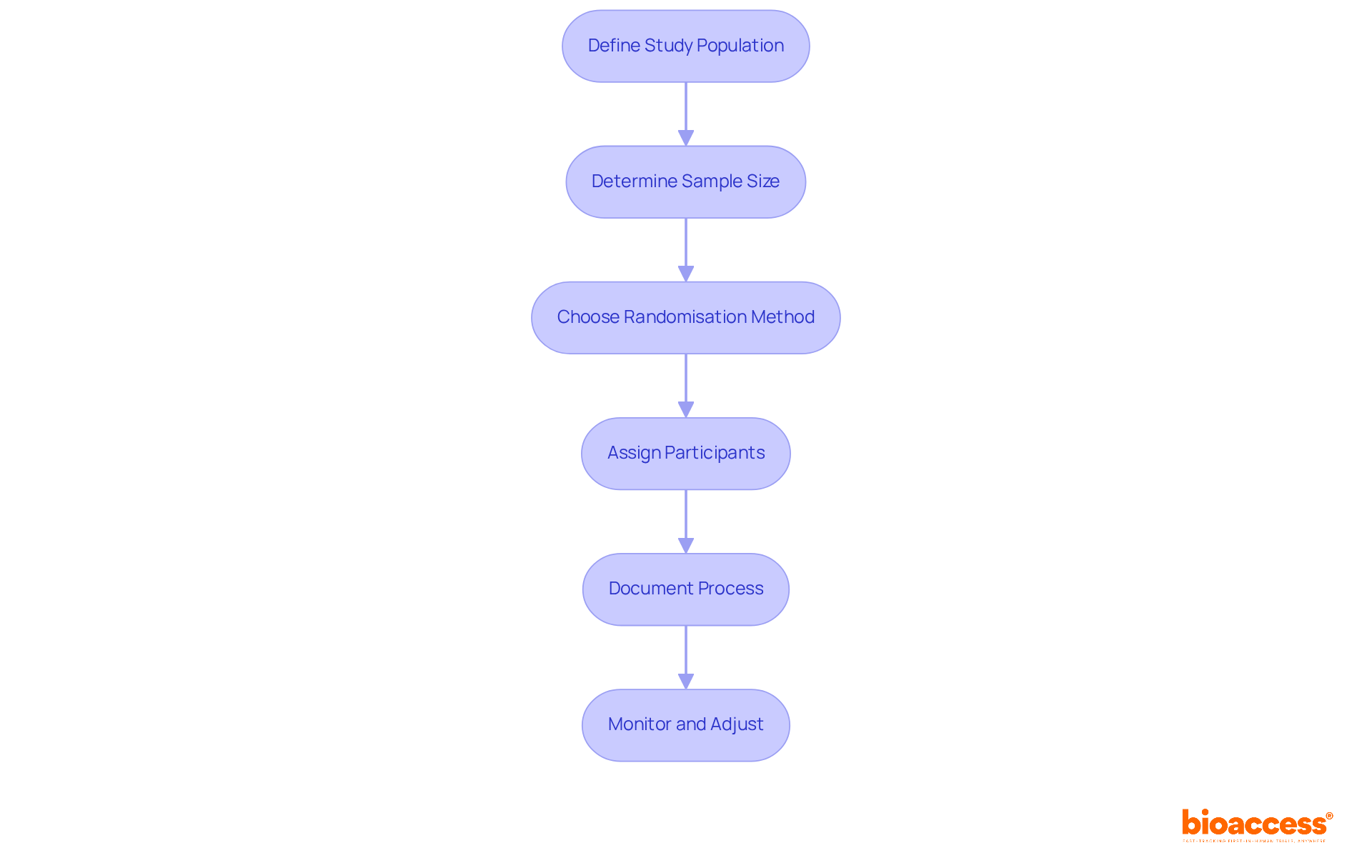
To implement simple randomisation in a clinical trial, it is essential to follow these systematic steps:
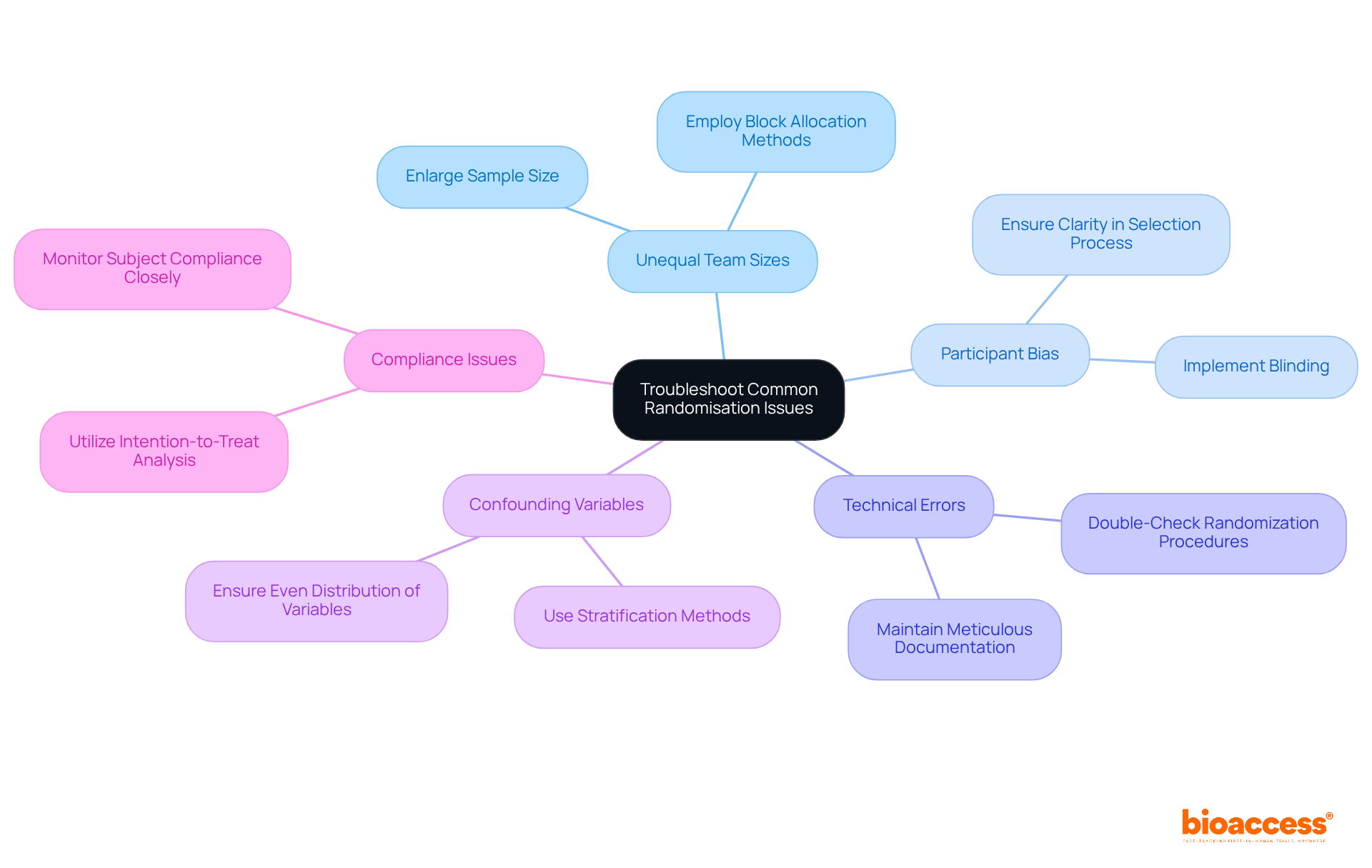
Implementing simple randomisation in clinical trials presents several challenges that researchers must navigate effectively. Bioaccess is here to support you through these complexities.
Unequal Team Sizes: Small trials often encounter disparities in team sizes due to the participant assignment process. Research indicates that simple randomisation can lead to significant imbalances, especially in small sample sizes. Notably, only 10% of simulations with 30 specimens demonstrated balanced groups. To mitigate this issue, consider enlarging the sample size or employing block allocation methods to maintain balance. Bioaccess assists in feasibility studies and site selection to ensure optimal sample sizes are achieved.
Participant Bias: Participants may develop perceptions about their treatment assignments based on personal preferences, which can introduce bias. As Nikolaos Pandis noted, 'Simple randomisation is a key step in reducing selection bias during the treatment allocation phase in randomized clinical trials.' To counteract this, ensure clarity in the selection process and implement blinding whenever possible, thus reducing the likelihood of bias influencing results. Our compliance assessments guarantee that the allocation process is transparent and adheres to regulatory standards.
Technical Errors: Mistakes in random number generation or manual assignment can compromise the integrity of the random selection process. It is crucial to double-check randomization procedures and maintain meticulous documentation to promptly identify and rectify any errors. Bioaccess offers project management services that encompass monitoring and reporting, assisting in preserving the integrity of your study.
Confounding Variables: Imbalances in essential traits between populations can jeopardize the validity of study outcomes. Using simple randomisation is an effective technique to ensure that critical variables are evenly distributed across treatment groups, thereby enhancing the robustness of the study. Our expertise in setting up evaluations ensures that these stratification methods are properly implemented.
Compliance Issues: Non-adherence to assigned treatments can skew results. The maximum decrease in test power with 20 specimens was 10% for large effects, highlighting the importance of closely monitoring subject compliance. Utilizing intention-to-treat analysis can help address dropouts or non-compliance in the final evaluation, ensuring that the study's findings remain valid and reliable. Bioaccess's study project management includes close monitoring of participant compliance to uphold the integrity of your research.
By addressing these common issues with strategic solutions and leveraging the comprehensive services offered by Bioaccess, researchers can enhance the effectiveness of their randomization methods and improve the overall quality of clinical trials.
Mastering simple randomisation in clinical trials is essential for ensuring unbiased participant allocation and maintaining the integrity of research findings. This straightforward yet powerful technique enhances the reliability and validity of studies, ultimately contributing to more accurate and applicable results in the medical field.
The article delves into the significance of simple randomisation, outlining its critical role in eliminating selection bias and ensuring comparability between treatment and control groups. Key steps for effective implementation are discussed, including:
Additionally, common challenges such as unequal team sizes and participant bias are addressed, along with strategic solutions to navigate these issues effectively.
In conclusion, embracing the principles of simple randomisation not only fortifies the ethical standards of clinical research but also empowers researchers to produce findings that can be confidently applied to broader populations. By adhering to best practices and remaining vigilant against common pitfalls, the potential for impactful and reliable clinical trials is significantly enhanced. The commitment to mastering randomisation techniques represents a vital step towards advancing medical knowledge and improving patient outcomes.
What is simple randomisation in clinical trials?
Simple randomisation is a technique used in clinical studies to allocate individuals to different treatment categories purely by chance, ensuring that each individual has an equal opportunity to be assigned to any category.
How is simple randomisation typically achieved?
Simple randomisation is typically achieved through straightforward methods, such as flipping a coin, to determine which treatment category an individual will join.
Why is simple randomisation important in clinical research?
Simple randomisation is important because it minimizes bias and establishes a fundamental methodology in clinical research, ensuring the integrity of study results.