


The Therapeutic Goods Administration (TGA) is crucial in ensuring that biopharmaceutical products adhere to rigorous safety and efficacy standards before entering the market. For biopharma companies, mastering TGA regulatory requirements is not merely a compliance necessity; it represents a strategic advantage that can expedite product approvals and stimulate innovation. However, as the biopharma landscape evolves, navigating the complexities of TGA regulations introduces distinct challenges.
How can companies effectively align their development strategies with these requirements to enhance their chances of success? This question is vital for fostering a proactive approach in clinical research, ensuring that organizations not only comply but thrive in a competitive environment.
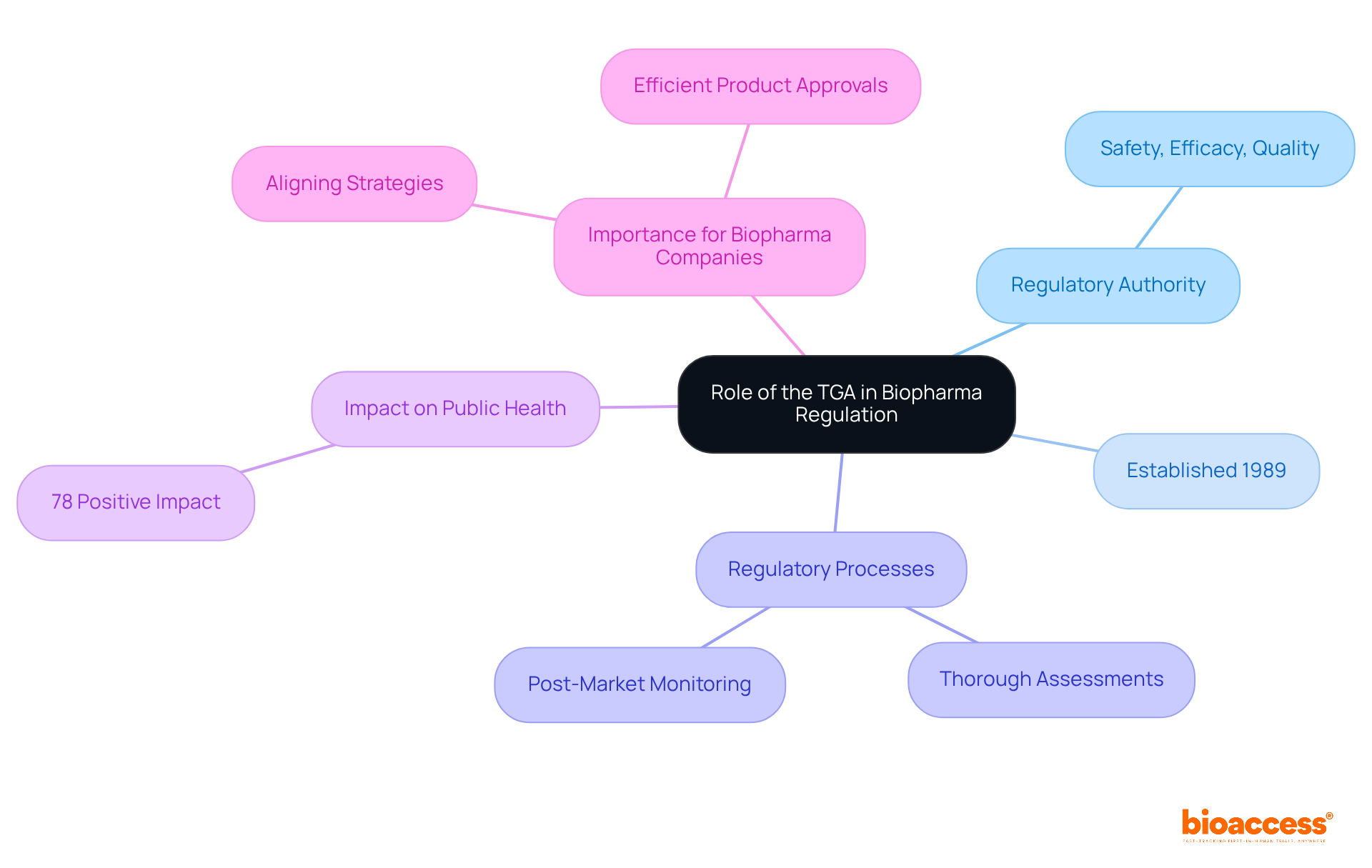
The Therapeutic Goods Administration (TGA) is Australia's regulatory authority, dedicated to ensuring the safety, efficacy, and quality of therapeutic goods, including medicines, medical devices, and biological products. Established under the Therapeutic Goods Act of 1989, the TGA enforces rigorous standards that all therapeutic goods must meet before entering the market. This process involves thorough assessments of research data, manufacturing practices, and ongoing post-market monitoring.
The TGA's oversight is vital for safeguarding public health, governing the entire lifecycle of therapeutic products - from initial development to market access and continuous compliance. Recent updates reveal that 78% of respondents reported a positive impact from the TGA's regulatory pathways. This statistic underscores the significance of these frameworks in facilitating timely access to innovative therapies.
For biopharma companies, it is essential to understand the TGA regulatory requirements for biopharma companies. It allows them to align their strategies with the TGA regulatory requirements for biopharma companies, ultimately leading to more efficient product approvals and successful market entry. As the Medtech landscape evolves, collaboration with the TGA becomes increasingly important to navigate challenges and leverage opportunities effectively.
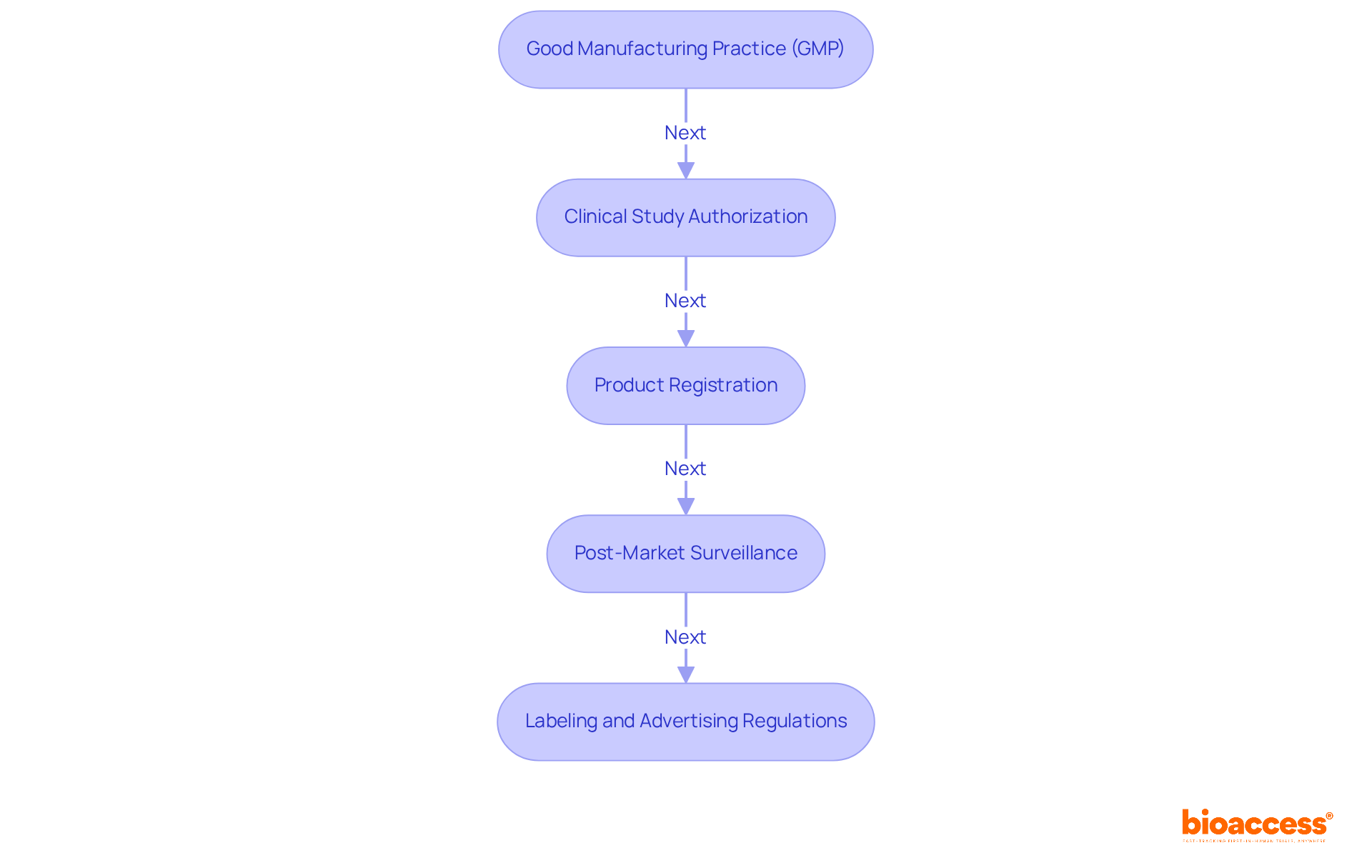
Biopharma companies must navigate the TGA regulatory requirements for biopharma companies, which are established by the TGA. Understanding the TGA regulatory requirements for biopharma companies is crucial for successful product development and market entry. Here are the key requirements:
Good Manufacturing Practice (GMP): Compliance with GMP is mandatory for all manufacturing processes to ensure product quality and safety. The TGA conducts regular inspections to verify adherence to these standards.
Clinical Study Authorization: Before commencing research studies, companies must submit a Clinical Study Notification (CSN) or Clinical Study Application (CSA) to the TGA, outlining the study's design, objectives, and safety measures. Bioaccess provides comprehensive clinical study management services, including feasibility assessments and site selection, to streamline this process.
Product Registration: Following successful tests, companies are required to register their products with the TGA, supplying detailed information on efficacy, safety, and quality. Bioaccess assists in ensuring that all necessary documentation meets TGA requirements.
Post-Market Surveillance: Ongoing monitoring of products post-approval is essential to ensure continued compliance and safety, including reporting adverse events. Bioaccess provides project management and reporting services to facilitate this process.
Labeling and Advertising Regulations: All promotional materials must comply with TGA guidelines to ensure they are not misleading and provide accurate information about the product. With expertise in regulatory affairs, Katherine Ruiz and the Bioaccess team ensure that all aspects of compliance are addressed effectively.
By effectively managing the TGA regulatory requirements for biopharma companies, they can significantly enhance their chances of successful product development and market entry.
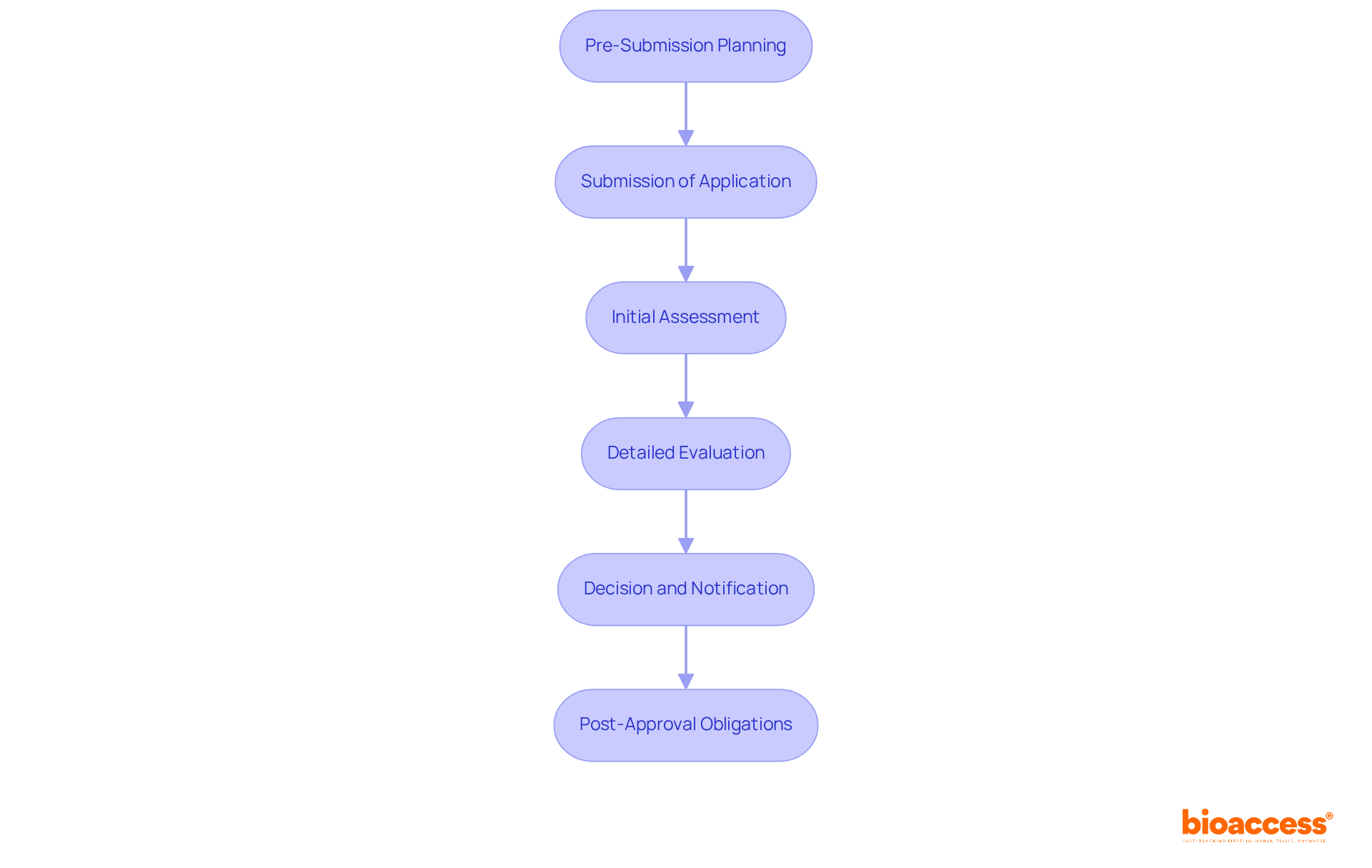
The TGA regulatory requirements for biopharma companies during the approval process for biopharmaceuticals are crucial for ensuring the safety and efficacy of new therapies. Understanding this process is essential for companies aiming to navigate the complexities of clinical research successfully while complying with TGA regulatory requirements for biopharma companies.
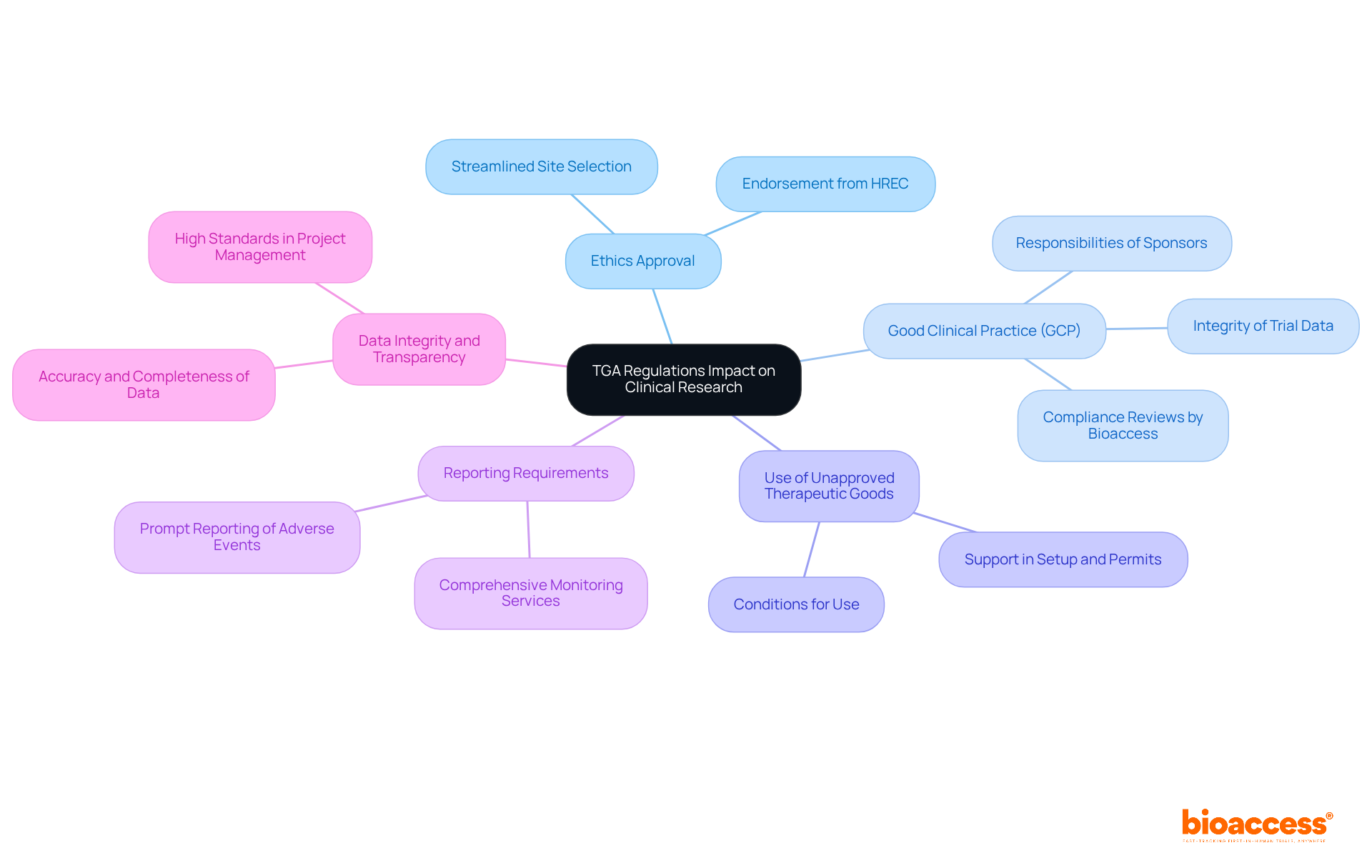
The TGA regulatory requirements for biopharma companies play a crucial role in shaping clinical research in Australia, influencing various aspects of study design and execution. Understanding the TGA regulatory requirements for biopharma companies is essential for researchers aiming to effectively navigate the complexities of the Medtech landscape.
Ethics Approval: Every medical study must secure endorsement from a Human Research Ethics Committee (HREC) before submission to the TGA. This process ensures that ethical standards are upheld. Bioaccess assists in the feasibility and selection of research sites and principal investigators (PIs), streamlining this essential step.
Adherence to Good Clinical Practice (GCP): Trials are mandated to follow GCP guidelines, which outline the responsibilities of sponsors, investigators, and monitors to safeguard the integrity of trial data. Bioaccess provides thorough reviews and feedback on study documents, ensuring compliance with national requirements.
Use of Unapproved Therapeutic Goods: The TGA allows the use of unapproved therapeutic goods in clinical studies under specific conditions, which must be clearly articulated in the study protocol. Bioaccess supports the setup and startup processes, including acquiring necessary import permits and nationalization of investigational devices.
Reporting Requirements: Researchers are obligated to promptly report any adverse events or safety concerns to the TGA, ensuring ongoing compliance and patient safety. Bioaccess offers comprehensive project management and monitoring services, including detailed reporting on study status and adverse events.
Data Integrity and Transparency: The TGA underscores the significance of data integrity, requiring that all data generated during clinical trials be accurate, complete, and verifiable. Bioaccess's commitment to high standards in project management ensures that data integrity and transparency are maintained throughout the research process.
In summary, collaboration with Bioaccess not only facilitates compliance with the TGA regulatory requirements for biopharma companies but also enhances the overall quality and integrity of clinical research.
Understanding the TGA regulatory requirements is crucial for biopharma companies aiming to navigate the complex landscape of therapeutic goods in Australia. The TGA ensures that all products meet stringent safety, efficacy, and quality standards, ultimately protecting public health and facilitating access to innovative therapies.
This article delves into the essential components of TGA regulations, highlighting the significance of:
It outlines the TGA approval process, emphasizing the importance of:
Furthermore, the impact of TGA regulations on clinical research is explored, underscoring the need for:
In conclusion, biopharma companies must prioritize a thorough understanding of TGA regulatory requirements to enhance their chances of successful product development and market entry. Engaging with the TGA streamlines the approval process and fosters a culture of compliance that benefits both the industry and public health. As the biopharmaceutical landscape evolves, embracing these regulatory frameworks is essential for driving innovation and ensuring the delivery of safe and effective therapies to patients.
What is the role of the TGA in biopharma regulation?
The TGA, or Therapeutic Goods Administration, is Australia's regulatory authority responsible for ensuring the safety, efficacy, and quality of therapeutic goods, including medicines, medical devices, and biological products.
When was the TGA established?
The TGA was established under the Therapeutic Goods Act of 1989.
What processes does the TGA use to regulate therapeutic goods?
The TGA enforces rigorous standards that therapeutic goods must meet before entering the market, which involves thorough assessments of research data, manufacturing practices, and ongoing post-market monitoring.
Why is the TGA's oversight important?
The TGA's oversight is crucial for safeguarding public health and governs the entire lifecycle of therapeutic products, from initial development to market access and continuous compliance.
What percentage of respondents reported a positive impact from the TGA's regulatory pathways?
Recent updates indicate that 78% of respondents reported a positive impact from the TGA's regulatory pathways.
How can biopharma companies benefit from understanding TGA regulatory requirements?
Understanding TGA regulatory requirements allows biopharma companies to align their strategies with these regulations, leading to more efficient product approvals and successful market entry.
Why is collaboration with the TGA becoming increasingly important for biopharma companies?
As the Medtech landscape evolves, collaboration with the TGA is essential for navigating challenges and leveraging opportunities effectively.