


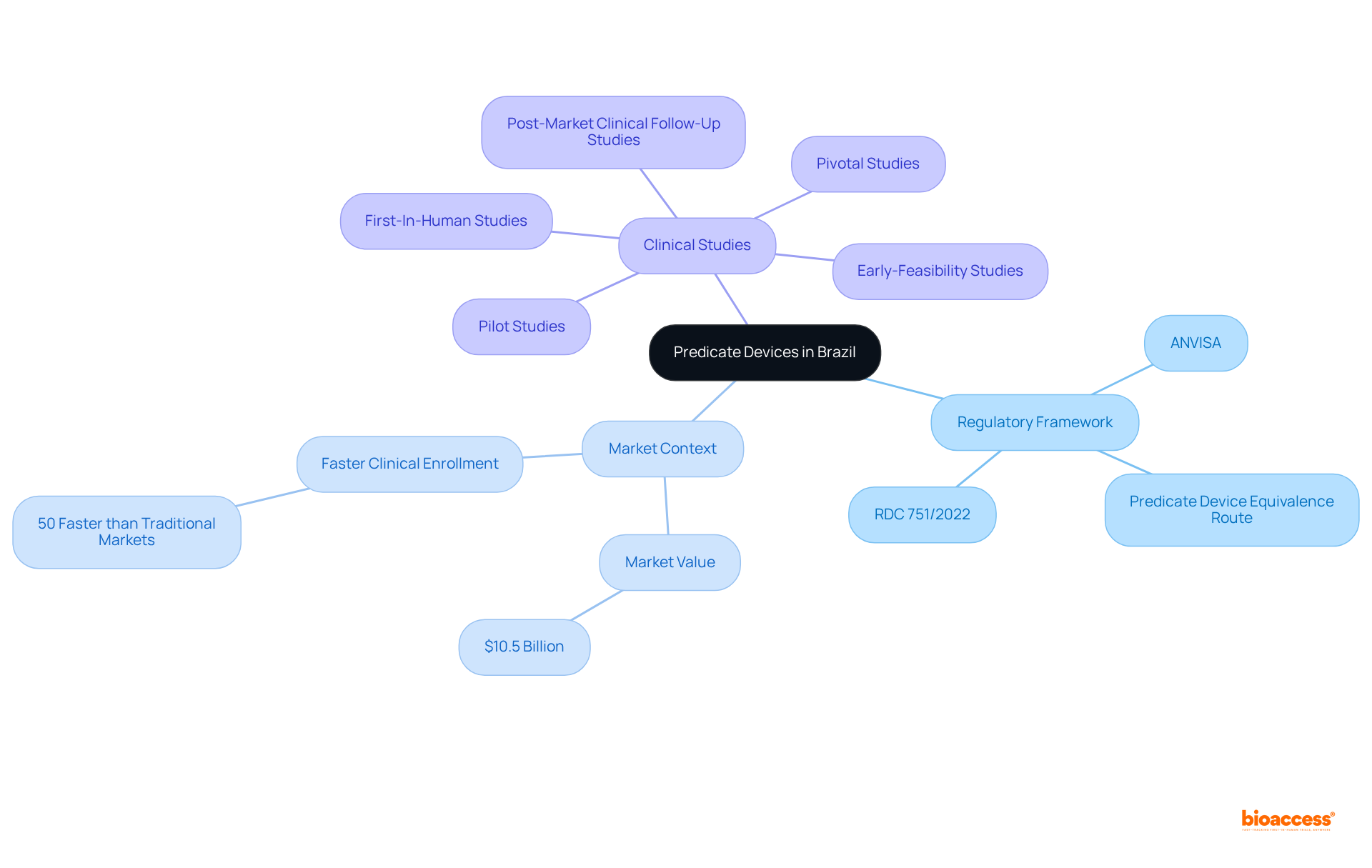
This article centers on the mastery of the Brazil predicate device equivalence route, a crucial pathway for regulatory approval of medical devices. Understanding predicate definitions is fundamental, as it lays the groundwork for establishing substantial equivalence. The article further delineates the necessary steps and best practices for navigating Brazil's regulatory landscape. It emphasizes that thorough documentation and collaboration with experts are critical components for a successful submission to ANVISA, thereby reinforcing the importance of strategic preparation in the regulatory process.
Navigating the complex landscape of medical device regulations in Brazil is essential for innovators aiming to penetrate a market valued at approximately $10.5 billion. The Brazil predicate device equivalence route presents a streamlined pathway for securing regulatory approval, facilitating faster market entry and enhancing competitiveness.
However, the challenge resides in mastering the intricacies of this process—how can one effectively identify and leverage predicate devices to ensure compliance and success?
This guide explores the essential steps and best practices for mastering the Brazil predicate device equivalence route, equipping professionals with the critical knowledge required to excel in this dynamic regulatory environment.
In Brazil, a classification instrument utilizes the brazil predicate device equivalence route as a medical tool previously authorized by the Brazilian Health Regulatory Agency (ANVISA), acting as a reference point for demonstrating the safety and effectiveness of new instruments. To qualify as a predicate, the apparatus must share the same intended use and technological characteristics as the new device. This understanding is crucial, underpinning the substantial equivalence claim necessary for regulatory approval. Knowledge of the pertinent regulations, particularly the Brazil predicate device equivalence route as outlined in RDC 751/2022, is essential as it specifies the classification and requirements for medical equipment in Brazil.
As of 2025, the authorization procedure for medical equipment in Brazil has undergone significant advancements, utilizing the brazil predicate device equivalence route which features an efficient pathway that allows for quicker approvals, typically within 4-6 weeks. The Brazilian medical equipment market is valued at approximately $10.5 billion, emphasizing the importance of understanding the brazil predicate device equivalence route in this regulatory landscape. Additionally, enrollment for clinical studies in Brazil is 50% faster than in traditional markets, rendering it an attractive option for innovators.
Leveraging the comprehensive clinical trial management services offered by bioaccess®, including expertise in:
will effectively guide you through the subsequent steps of the registration process. This collaboration ensures compliance and facilitates market entry, positioning you for success in the dynamic Medtech environment.
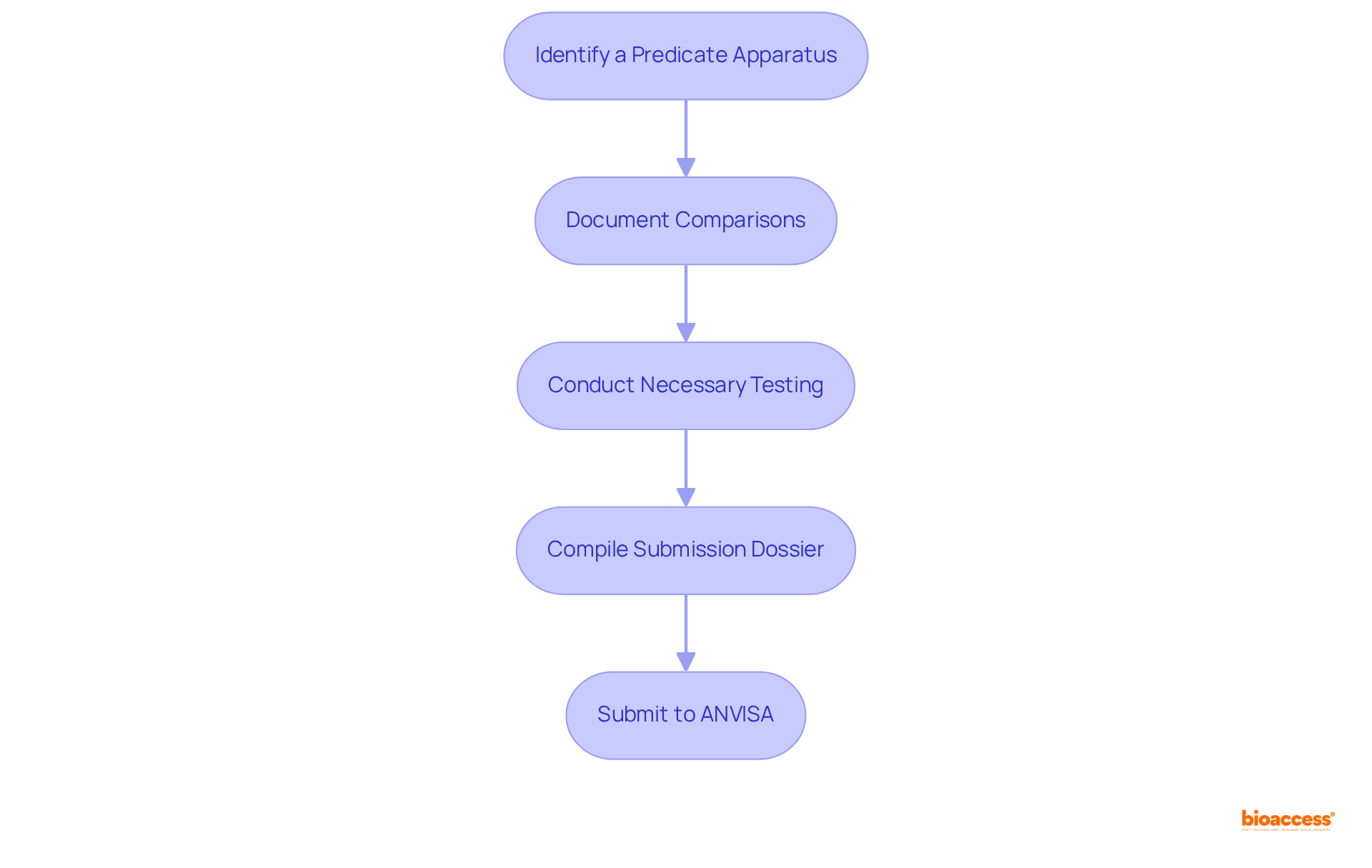
To establish substantial equivalence in Brazil, it is essential to follow these key criteria and steps:
Identify a Predicate Apparatus: Start by researching existing tools that closely resemble your new apparatus. Utilize databases such as ANVISA's or the FDA's 510(k) database to identify similar products that align with your item's intended use and technological features.
Document Comparisons: Prepare a comprehensive comparison of your equipment against the recognized reference apparatus. Highlight similarities in intended use, design, materials, and performance metrics to substantiate your claims effectively.
Conduct Necessary Testing: If required, perform testing to demonstrate that your apparatus meets or exceeds the performance of the reference equipment. This may include bench testing, biocompatibility assessments, or clinical evaluations to confirm the safety and efficacy of your product.
Compile Submission Dossier: Assemble a detailed submission dossier that includes all relevant documentation, test results, and comparative analyses. Ensure compliance with ANVISA's guidelines for medical device submissions via the brazil predicate device equivalence route to facilitate a seamless evaluation. Notably, ethical approvals in Brazil are typically granted within 4-6 weeks, underscoring the efficiency of the submission process. Engaging a prominent contract research organization, such as bioaccess, can streamline this procedure, ensuring meticulous management of trial setup, compliance evaluations, and project oversight, including feasibility assessments and site selection.
Submit to ANVISA: Finally, submit your dossier to ANVISA for evaluation. Be prepared to address any inquiries or requests for additional information that may arise during the review process, ensuring timely communication to expedite approval. As emphasized by industry specialists, comprehensive documentation and adherence to the brazil predicate device equivalence route are crucial for a successful submission. Collaborating with experts such as Katherine Ruiz, a recognized authority in regulatory matters for medical products and in vitro diagnostics in Colombia, can provide valuable insights into navigating the complexities of the regulatory environment in Latin America.
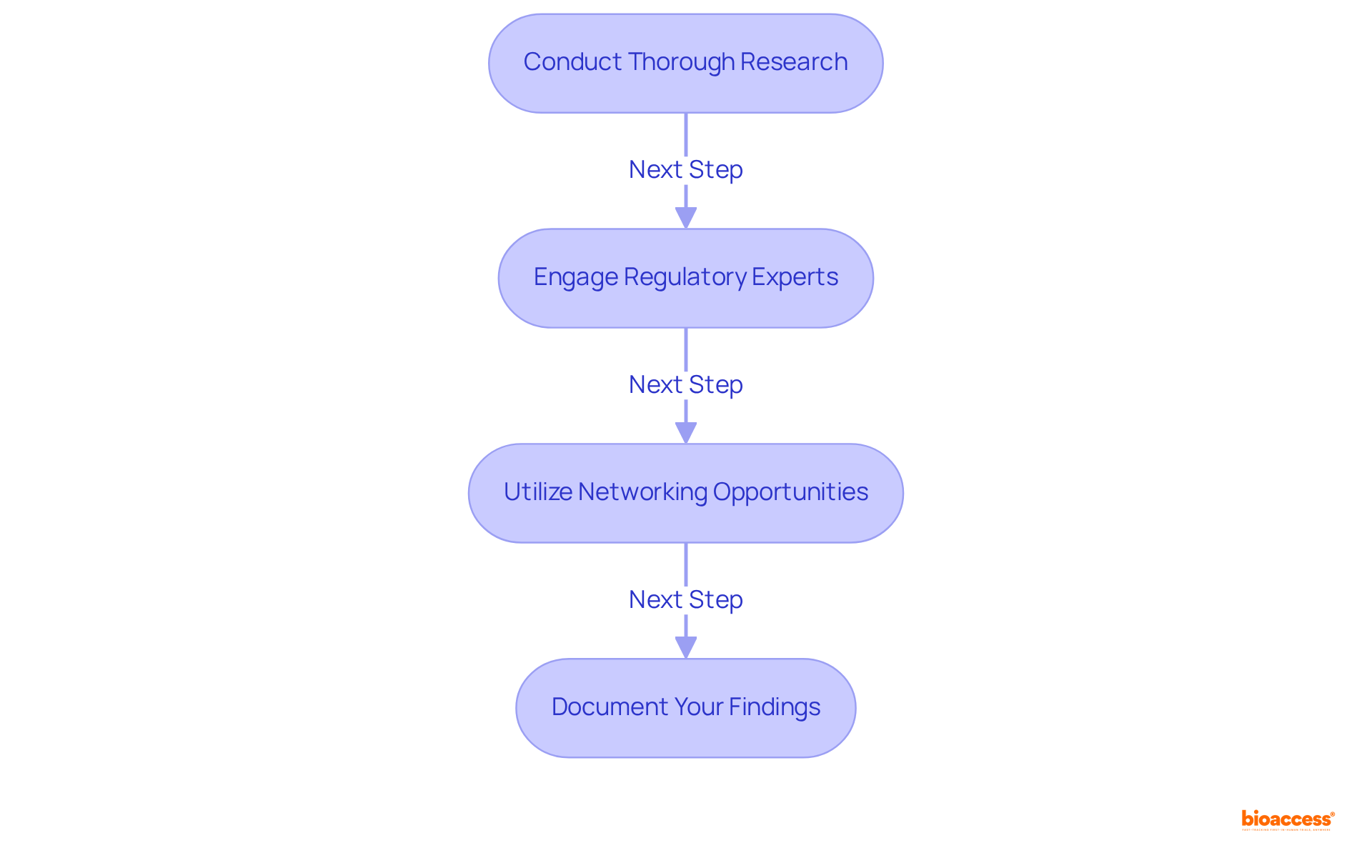
Recognizing the appropriate reference equipment can present various challenges in clinical research. To effectively navigate these hurdles, consider the following strategies:
Conduct Thorough Research: Leverage multiple resources, including ANVISA's database, the FDA's 510(k) database, and industry publications. This comprehensive approach enhances your ability to identify potential predicate tools effectively, ensuring you have a well-rounded view of available options.
Engage Regulatory Experts: Collaborate with regulatory affairs professionals or contract research organizations (CROs) specializing in Brazilian medical equipment regulations. Their expertise can offer essential insights and enhance the identification method via the brazil predicate device equivalence route, significantly increasing your chances of success. With over 15 years of experience, bioaccess is well-positioned to assist in navigating these complexities, providing services such as feasibility studies, site selection, compliance reviews, trial setup, import permits, project management, and reporting.
Utilize Networking Opportunities: Participate in industry conferences, webinars, and workshops to connect with other professionals. Networking can yield valuable suggestions for relevant tools that may not be easily obtainable through conventional research methods, thereby broadening your resource pool.
Document Your Findings: Maintain thorough records of your research methodology, including the rationale for selecting specific equipment. This documentation will prove invaluable during the submission process, particularly in addressing inquiries from ANVISA, and demonstrates a thorough approach to compliance.
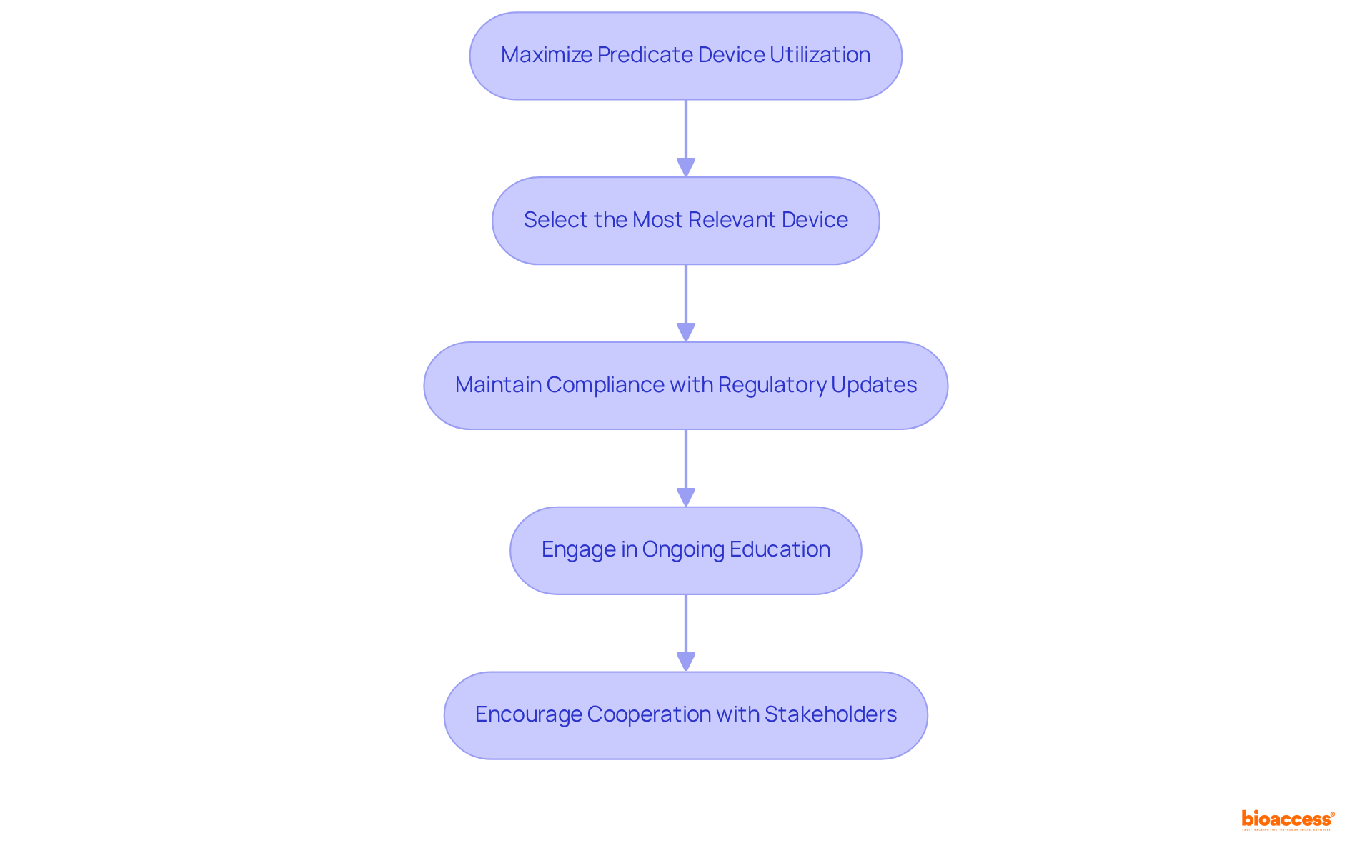
To maximize the effectiveness of predicate device utilization, it is essential to consider the following best practices:
Select the Most Relevant Device: Choose a device that closely aligns with your new apparatus regarding intended use and technological characteristics. A closer match simplifies the demonstration of substantial equivalence, facilitating a smoother regulatory procedure. For instance, Brazil's ANVISA permits local comparisons that emphasize product classification and clinical evaluation, which can streamline your submission process via the brazil predicate device equivalence route.
Maintain Compliance with Regulatory Updates: Staying informed about any changes in ANVISA regulations or guidelines regarding predicate products is crucial. Consistently examining updates from ANVISA and other governing organizations ensures ongoing compliance and readiness to adapt to evolving requirements. Understanding INVIMA's role in Colombia, which supervises medical instruments and is recognized as a Level 4 health authority by PAHO/WHO, provides valuable insights into compliance expectations and best practices in the region.
Engage in Ongoing Education: Participation in training sessions and workshops focused on compliance with regulations and predicate equipment usage is vital. This ongoing education keeps you and your team abreast of best practices and emerging trends, enhancing your strategic approach. Insights from professionals such as Ana Criado, with extensive experience in compliance matters and biomedical engineering, can deepen your understanding of the intricacies associated with medical equipment regulations.
Encourage Cooperation with Stakeholders: Establishing robust connections with stakeholders, including compliance advisors, clinical researchers, and manufacturers, is essential. Collaborative efforts can lead to shared understandings and improved strategies for effectively employing specific tools, ultimately enhancing adherence and expediting market entry. Additionally, leveraging technology and regulatory intelligence platforms can assist in identifying suitable predicate devices and preparing efficient submissions.
Mastering the Brazil predicate device equivalence route is essential for innovators aiming to navigate the intricate landscape of medical device regulation in Brazil. Understanding the criteria for substantial equivalence and the streamlined approval process enables stakeholders to effectively position their products for success in a burgeoning market valued at approximately $10.5 billion.
Key insights into:
underscore the necessity of thorough research and collaboration with regulatory experts. Engaging with resources such as ANVISA's database and leveraging the expertise of organizations like bioaccess can significantly enhance the likelihood of successful device approval.
Ultimately, embracing best practices for predicate device utilization is crucial for ensuring compliance and facilitating market entry. Staying informed about regulatory updates, fostering stakeholder cooperation, and committing to ongoing education will empower innovators to overcome challenges and capitalize on the opportunities within Brazil's dynamic Medtech environment. A proactive approach to mastering the predicate device equivalence route not only streamlines the approval process but also positions companies to thrive in a competitive landscape.
What are predicate devices in Brazil's regulatory context?
Predicate devices in Brazil are medical tools previously authorized by the Brazilian Health Regulatory Agency (ANVISA) that serve as reference points for demonstrating the safety and effectiveness of new medical instruments.
What criteria must a device meet to qualify as a predicate in Brazil?
To qualify as a predicate, a device must share the same intended use and technological characteristics as the new device.
Why is understanding the predicate device equivalence route important?
Understanding the predicate device equivalence route is crucial for making substantial equivalence claims necessary for regulatory approval in Brazil.
What does RDC 751/2022 specify?
RDC 751/2022 outlines the classification and requirements for medical equipment in Brazil, including the predicate device equivalence route.
How has the authorization procedure for medical equipment in Brazil changed as of 2025?
The authorization procedure has become more efficient, allowing for quicker approvals typically within 4-6 weeks through the predicate device equivalence route.
What is the estimated value of the Brazilian medical equipment market?
The Brazilian medical equipment market is valued at approximately $10.5 billion.
How does the enrollment for clinical studies in Brazil compare to traditional markets?
Enrollment for clinical studies in Brazil is 50% faster than in traditional markets, making it an attractive option for innovators.
What services does bioaccess® offer to assist with the registration process?
Bioaccess® offers comprehensive clinical trial management services, including expertise in early-feasibility studies, first-in-human studies, pilot studies, pivotal studies, and post-market clinical follow-up studies.
How can collaboration with bioaccess® benefit innovators in the Medtech environment?
Collaboration with bioaccess® ensures compliance with regulations and facilitates market entry, positioning innovators for success in the dynamic Medtech environment.