


The article emphasizes the effective utilization of the FDA database for medical devices, underscoring its critical role in ensuring compliance and facilitating market access. It elaborates on the database's functionalities, including device classifications and regulatory requirements, while providing a step-by-step guide for navigating the database. These elements are essential for stakeholders in the Medtech industry, enabling them to streamline product development and approval processes.
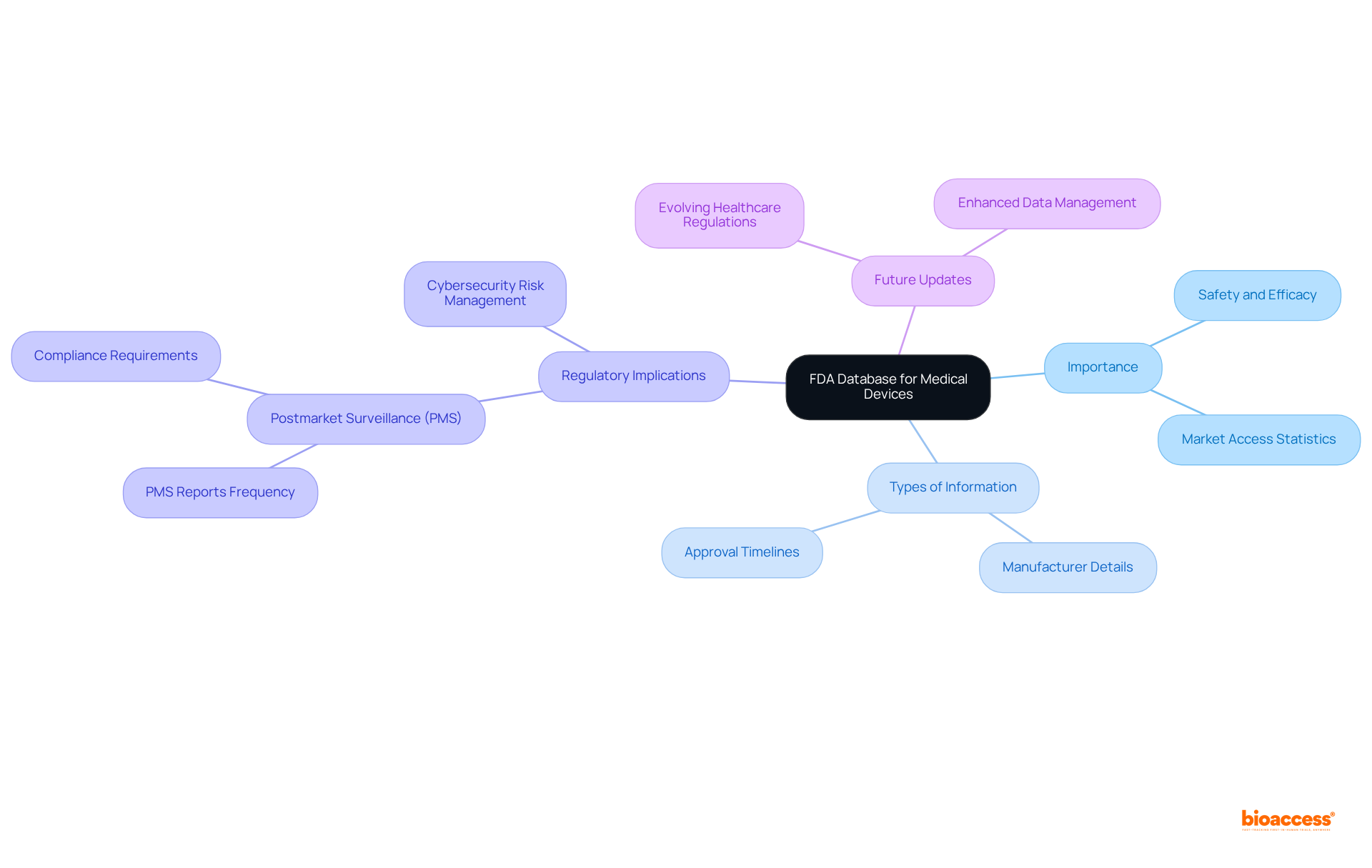
The FDA database for medical devices serves as a cornerstone of safety and regulatory compliance within the healthcare industry, encapsulating vital information that influences the development and marketing of medical technologies. By exploring this comprehensive repository, stakeholders can uncover critical insights into:
This knowledge ultimately enhances their strategic positioning in a competitive landscape. However, in light of the evolving regulatory environment and the increasing complexity of medical device classifications, how can professionals effectively navigate this intricate system to ensure both compliance and market readiness?
The FDA database medical devices serves as an extensive repository of information regarding healthcare instruments, including their classifications, approval statuses, and regulatory requirements. This database plays a crucial role in ensuring that medical equipment meets safety and efficacy standards prior to market entry. By exploring the FDA database medical devices, stakeholders can access critical data that informs their research, development, and marketing strategies. This overview will address the various elements of the FDA database medical devices, including summary information, manufacturer details, and approval timelines, all of which are essential for compliance and strategic planning within the Medtech industry.
Prompt access to the FDA database medical devices is paramount, especially considering that ethical approvals in the EU can be granted within 4-6 weeks. This timeframe underscores the effectiveness of oversight pathways available to stakeholders. Additionally, the requirement for postmarket surveillance (PMS) is integral to the FDA's regulatory framework, ensuring the continuous safety and effectiveness of health products once they are available to consumers. As of 2025, the FDA database medical devices will be updated to reflect evolving healthcare product regulations, particularly emphasizing the importance of comprehensive cybersecurity risk management programs, which are vital for compliance and safety in the Medtech sector. The influence of the database extends to market access statistics, where timely access to information can significantly reduce time-to-market and enhance competitive positioning within the healthcare landscape.
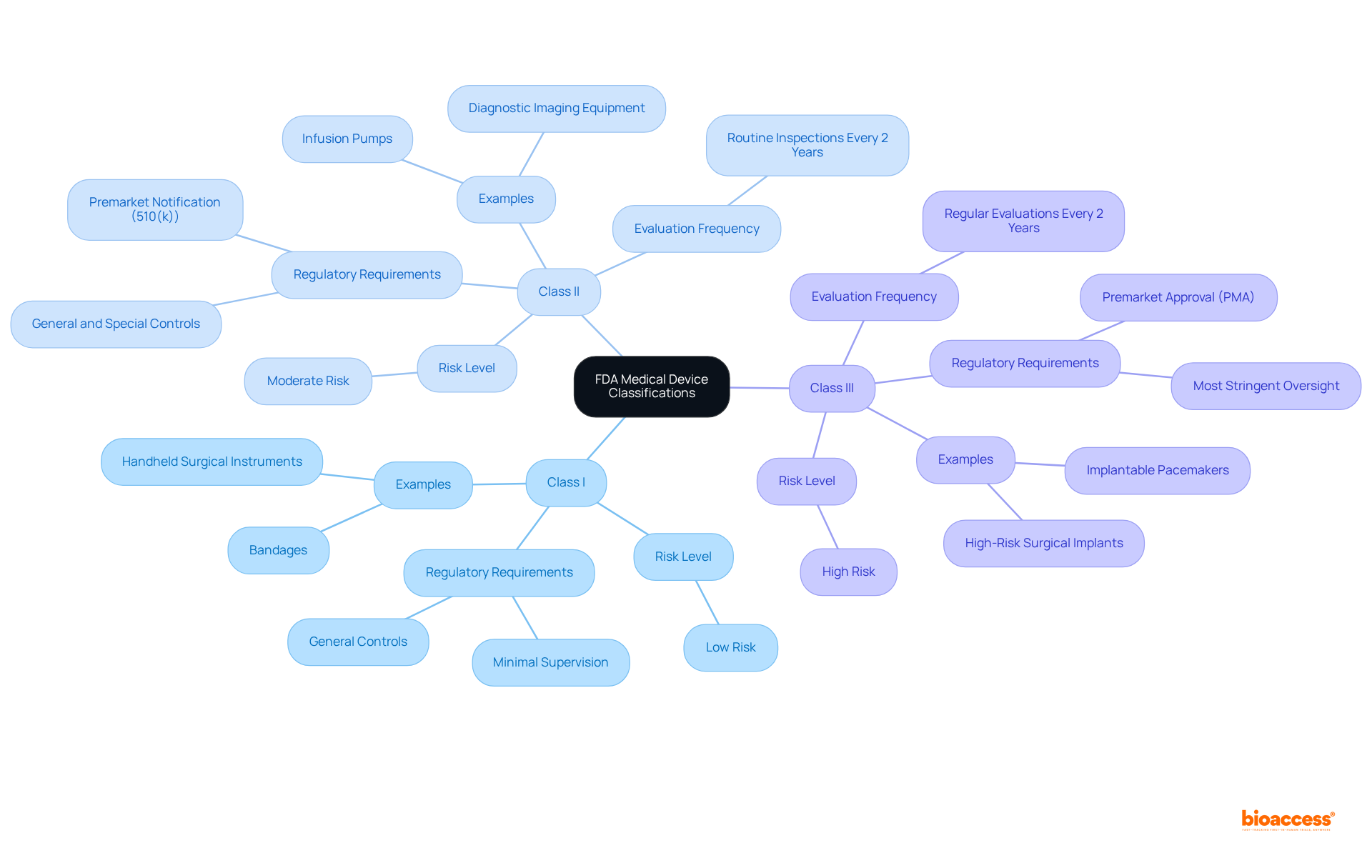
The FDA classifies medical instruments into three primary categories: Class I, Class II, and Class III, based on their associated risk levels.
Understanding these classifications is crucial for manufacturers, as they dictate the specific premarket submissions and compliance requirements necessary for each type of equipment. The FDA database medical devices serves as a valuable resource, offering detailed insights into each item's classification, product codes, and compliance requirements. As of 2025, the FDA continues to streamline its review processes, enhancing the speed and efficiency of product approvals while ensuring patient safety. This ongoing evolution in oversight pathways underscores the importance of remaining informed about classification standards and their implications for product development and market access. Furthermore, it is essential to recognize that the FDA conducts regular evaluations for Class II and III medical products every two years, with manufacturers typically receiving five days' notice prior to these evaluations. Additionally, products that are not substantially equivalent to cleared Class I, II, or III items may qualify for the De Novo process, providing a more flexible regulatory pathway.
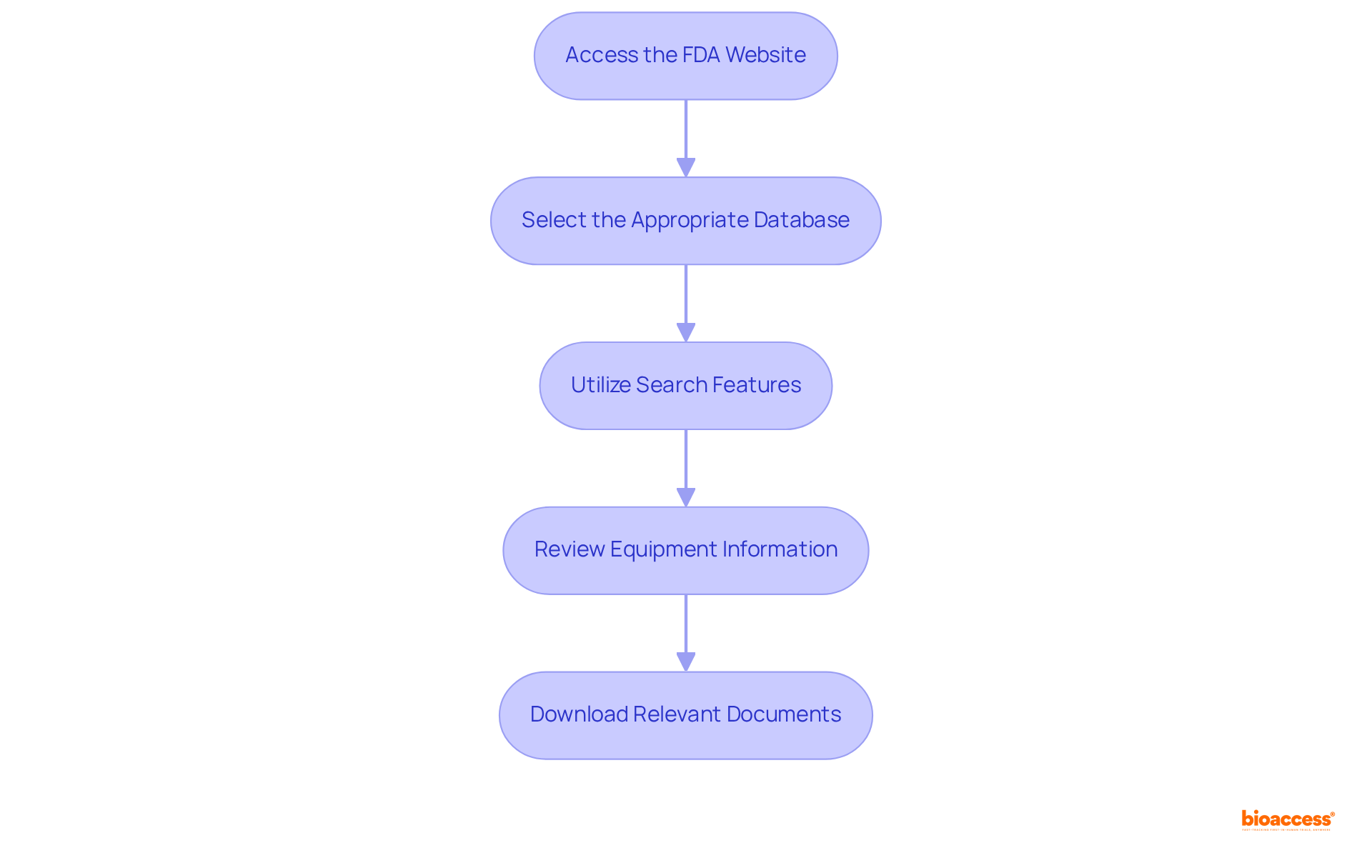
To navigate the FDA database effectively, follow these steps:
By following these steps, you can efficiently navigate the FDA database for medical devices, enhancing your ability to access critical information for successful approvals. Interacting with compliance experts or consultants can also offer valuable insights and guidance throughout the submission process.
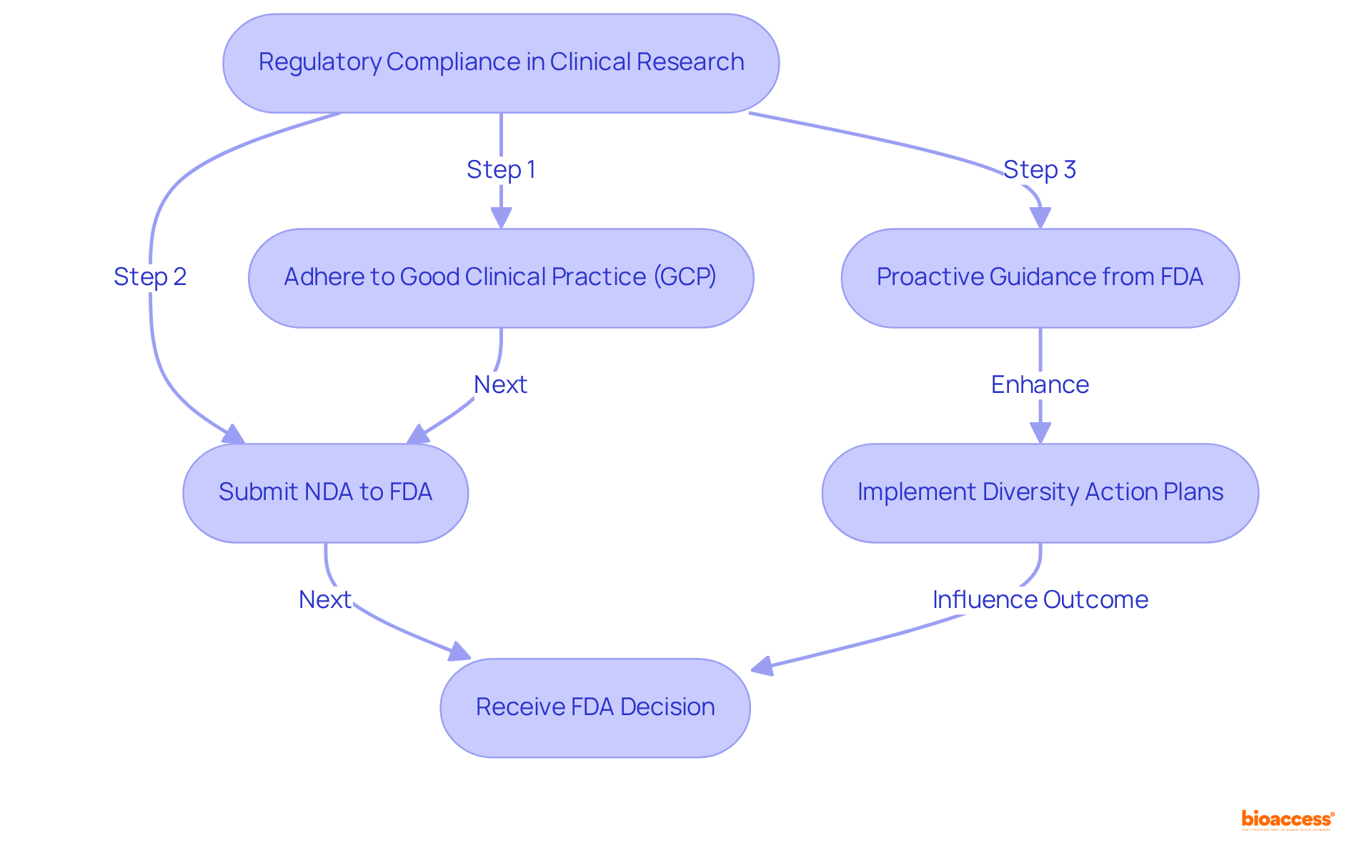
Regulatory compliance is paramount in clinical research within the healthcare equipment sector, ensuring that studies adhere to established protocols that protect patient safety and uphold data integrity. Compliance with FDA regulations requires strict adherence to Good Clinical Practice (GCP) standards, which govern the design, conduct, and reporting of clinical trials. Non-compliance can result in significant delays in product approval, increased costs, and potential legal ramifications.
For example, the FDA has 60 days to decide whether to file a New Drug Application (NDA) for review, with the review team allotted six to ten months to evaluate the submitted data regarding a drug's safety and effectiveness. The FDA's emphasis on early involvement during study design can streamline the approval process; sponsors who proactively seek guidance often experience more seamless interactions with regulatory authorities.
Additionally, the FDA's recent initiatives, including the requirement for Diversity Action Plans (DAPs), aim to enhance participant diversity in clinical trials, thereby improving the applicability of study results to broader populations. This proactive compliance approach not only strengthens the credibility and reliability of research findings but also significantly influences clinical trial success rates.
Adhering to GCP principles is essential; studies that exemplify these practices demonstrate improved results and approval, ultimately fostering the development of innovative medical devices. With over 15 years of experience, bioaccess® is uniquely positioned to navigate clients through these regulatory complexities, ensuring compliance and enhancing the likelihood of successful clinical trials.
The FDA database for medical devices stands as an indispensable resource for stakeholders within the healthcare industry, providing essential information that guarantees compliance and safety for medical equipment. Mastering this database not only facilitates informed decision-making but also enhances the overall efficiency of product development and market access, ultimately benefiting patient care.
Throughout this article, we have presented key insights regarding the significance of understanding medical device classifications, effectively navigating the FDA database, and adhering to regulatory compliance. The distinctions between Class I, II, and III devices illustrate the varying levels of risk and regulatory scrutiny, while the step-by-step guide offers a practical approach to accessing critical information. Furthermore, the emphasis on compliance with Good Clinical Practice standards underscores the vital importance of regulatory adherence in clinical research.
In light of the evolving landscape of medical device regulation, it is imperative for professionals in the Medtech industry to remain informed about the FDA database and its implications. By leveraging this resource, stakeholders can streamline their processes and contribute to the advancement of safe and effective medical technologies. Embracing the tools and knowledge available through the FDA database will empower healthcare professionals to navigate the complexities of the industry with both confidence and precision.
What is the FDA database for medical devices?
The FDA database for medical devices is an extensive repository that provides information regarding healthcare instruments, including their classifications, approval statuses, and regulatory requirements.
Why is the FDA database important?
The FDA database is crucial for ensuring that medical equipment meets safety and efficacy standards before entering the market, and it helps stakeholders access critical data for their research, development, and marketing strategies.
What types of information can be found in the FDA database?
The FDA database includes summary information, manufacturer details, approval timelines, and other elements essential for compliance and strategic planning within the Medtech industry.
How does the FDA database impact market access?
Timely access to information from the FDA database can significantly reduce time-to-market for medical devices and enhance competitive positioning within the healthcare landscape.
What is the significance of postmarket surveillance (PMS) in the FDA's regulatory framework?
Postmarket surveillance is integral to the FDA's regulatory framework as it ensures the continuous safety and effectiveness of health products once they are available to consumers.
What changes are expected in the FDA database by 2025?
By 2025, the FDA database will be updated to reflect evolving healthcare product regulations, with an emphasis on comprehensive cybersecurity risk management programs vital for compliance and safety in the Medtech sector.
How quickly can ethical approvals in the EU be granted, and what does this imply for the FDA database?
Ethical approvals in the EU can be granted within 4-6 weeks, highlighting the importance of prompt access to the FDA database for stakeholders to navigate regulatory pathways effectively.