


This article serves as a comprehensive guide for researchers, detailing how to effectively navigate and utilize the FDA Devices Database. This resource is essential for accessing information on medical instruments authorized for use in the United States. By outlining the steps necessary for accessing the database, leveraging search features, interpreting device data, and remaining informed about updates, the article highlights the critical importance of mastering these resources. Such expertise not only enhances research outcomes but also ensures compliance with regulatory standards.
Navigating the complexities of medical device regulation presents a significant challenge for researchers, particularly in comprehending the extensive FDA Devices Database. This vital resource catalogs thousands of authorized medical instruments, playing a pivotal role in ensuring compliance and enhancing research outcomes. Given the rapid evolution of regulatory frameworks and the overwhelming volume of information available, researchers must ask: how can they effectively leverage this database to drive innovation and ensure patient safety? This guide provides a step-by-step approach to mastering the FDA Devices Database, equipping researchers with essential tools to streamline their inquiries and make informed decisions within the fast-paced MedTech landscape.
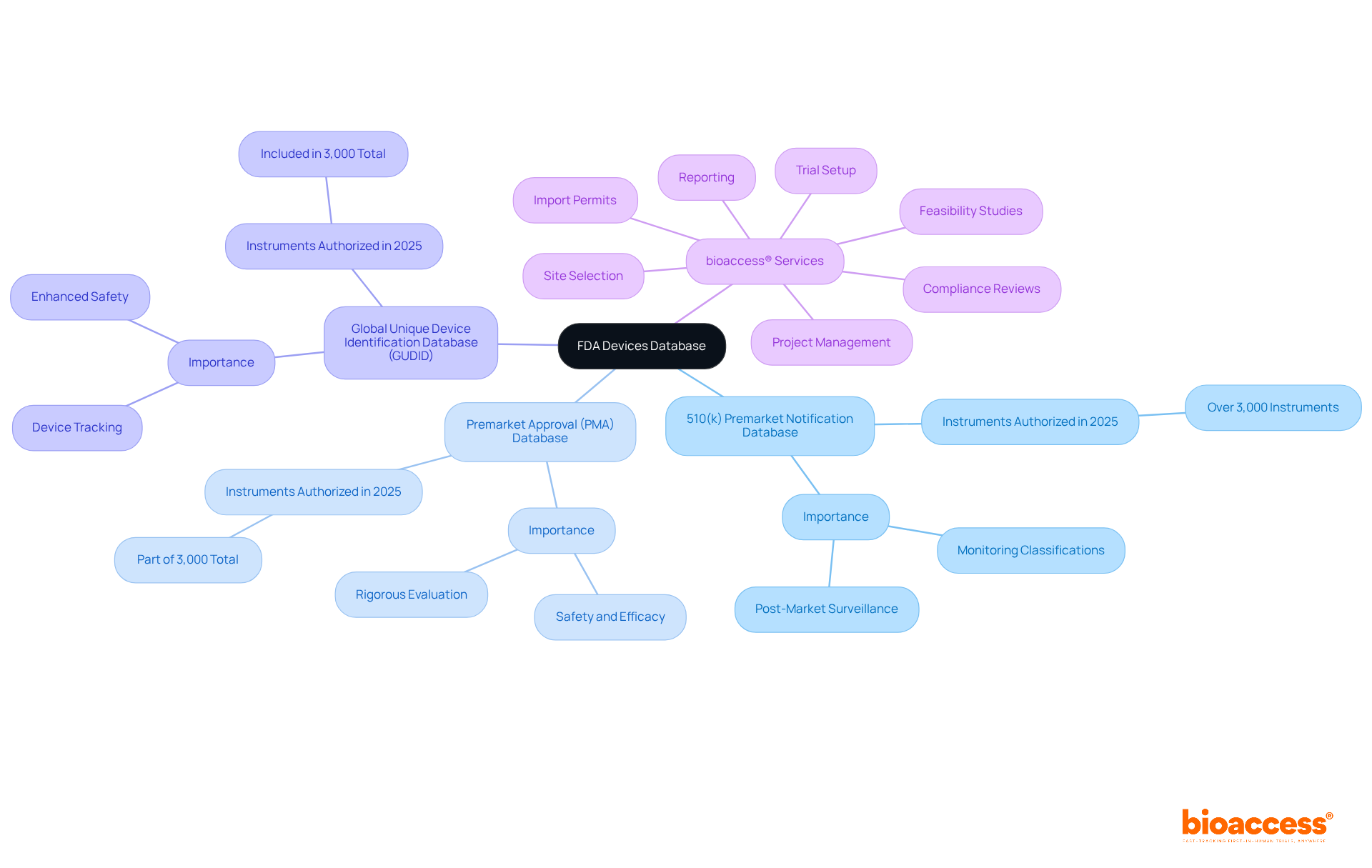
The FDA Equipment Registry serves as a vital resource, cataloging medical instruments authorized for use in the United States. This registry includes several key databases, such as:
Each of these databases is crucial for monitoring classifications, approvals, and post-market surveillance. Notably, in 2025 alone, the FDA authorized over 3,000 medical instruments, highlighting the database's importance in clinical research. A thorough understanding of the structure and function of these components is essential for researchers, as it empowers them to efficiently locate and interpret device information relevant to their studies.
As industry leaders emphasize, navigating the FDA devices database transcends mere compliance; it involves leveraging this information to enhance research outcomes and drive innovation in the MedTech field. Familiarity with the FDA devices database ultimately enables researchers to meet compliance requirements while advancing their research objectives.
Moreover, partnering with bioaccess® can offer comprehensive clinical trial management services, encompassing:
With over 20 years of experience overseeing Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies (PMCF), bioaccess® equips researchers with the essential tools and knowledge to adeptly navigate the complexities of clinical trials and regulatory environments, thereby enhancing their ability to utilize the FDA devices database effectively.
To access the FDA Devices Database, follow these steps:
Navigating the FDA Equipment Database can be complicated, particularly given that governance structures for medical apparatus are continually changing. Understanding the most accessed databases, particularly the FDA Devices Database, can streamline your research; for instance, the 510(k) database is frequently utilized for its comprehensive information on device clearances. Experts in the field, such as Ana Criado, Director of Compliance Affairs and a professor with vast experience in biomedical engineering and health economics, stress that "compliance is not merely a hurdle to be cleared; it’s a continuous commitment to patient safety and product quality." This perspective underscores the importance of mastering the FDA's resources to ensure adherence to evolving regulatory frameworks.

To effectively utilize the search features in the FDA Devices Database, it is essential to follow these steps:
Researchers frequently employ an average of five to seven search parameters when navigating these databases, highlighting the complexity of the governing landscape. It is crucial to keep in mind that the FDA devices database includes new items in the 510(k) database around the 5th of each month for products approved in the previous month when organizing your searches. Given that the current regulatory search process is time-consuming and prone to error, a streamlined approach to searching can significantly reduce the time spent on regulatory inquiries, allowing for more efficient decision-making. By mastering these search features, you can enhance your understanding of the FDA's classification processes and improve your product development strategies.
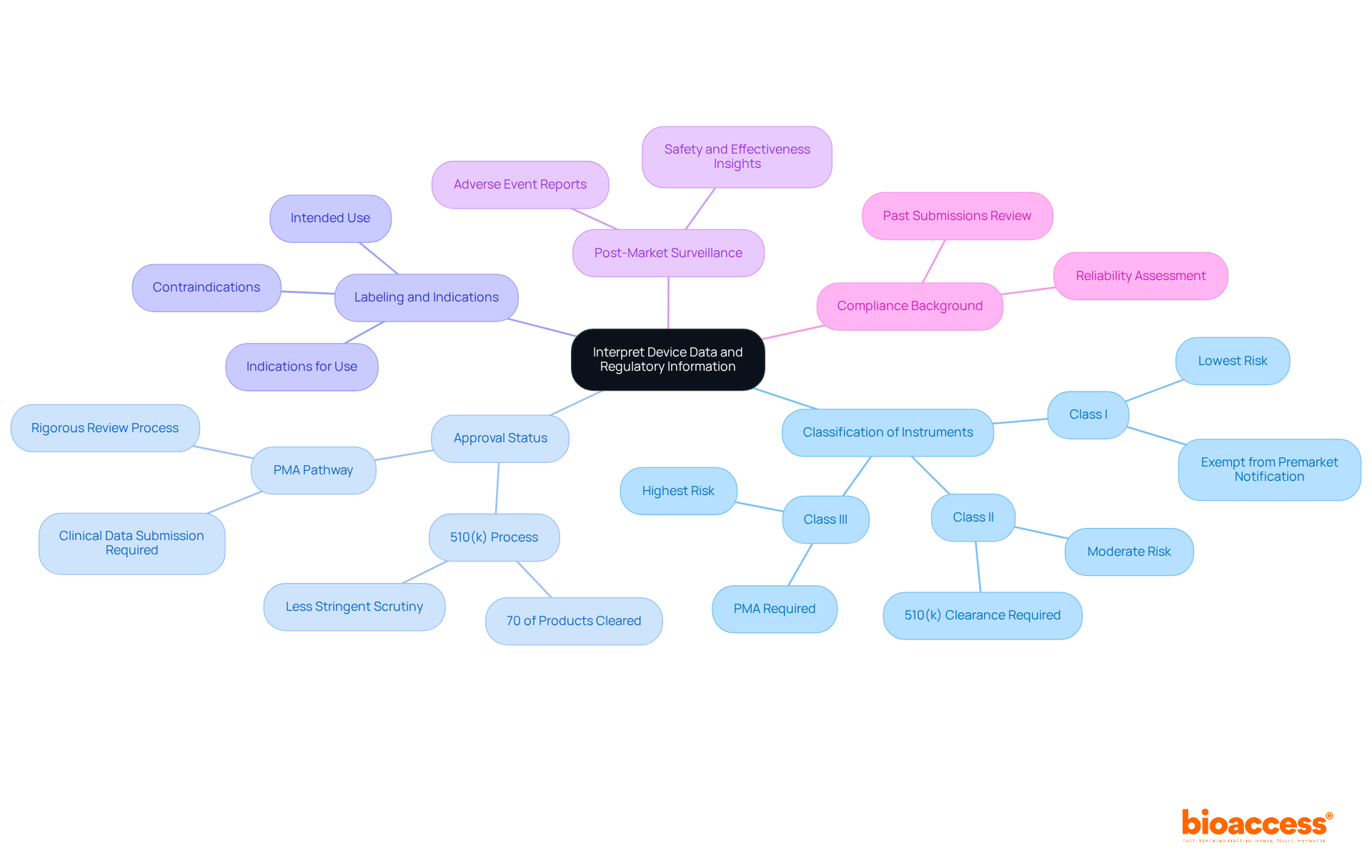
Interpreting device data from the fda devices database is essential for understanding the complexities of medical instruments and their regulatory landscape.
Classification of Instruments: Medical instruments are categorized into three classes (I, II, or III) based on their risk levels. Class I products generally pose the lowest risk and are often exempt from premarket notification, while Class III items, which support or sustain human life, require a more rigorous Premarket Approval (PMA) process due to their higher risk. Familiarizing yourself with these classifications is essential for assessing the regulatory requirements listed in the fda devices database.
Approval Status: It is crucial to determine whether a product has been cleared through the 510(k) process or approved via the PMA pathway. In 2025, approximately 70% of products submitted to the FDA devices database were cleared through the 510(k) process, indicating that a substantial number of items undergo less stringent scrutiny compared to those requiring PMA.
Labeling and Indications: Review the product's labeling information, which outlines its intended use, indications for use, and any contraindications. This information is crucial for comprehending the extent of the application's use and ensuring adherence to the regulations found in the FDA devices database.
Post-Market Surveillance: Investigate any post-market studies or adverse event reports related to the fda devices database for the product. These reports can offer valuable insights into the product's safety and effectiveness, highlighting any potential risks that may arise after market entry.
Compliance Background: Grasping the compliance background of a product can assist in assessing its reliability and the FDA's trust in its performance. A thorough review of past submissions and approvals can inform future development strategies and risk assessments.
To stay informed about updates and changes to the FDA Devices Database, consider implementing the following strategies:
By actively engaging with these resources, researchers can enhance their understanding of FDA regulations and ensure compliance, ultimately contributing to improved patient outcomes and device safety.
Mastering the FDA Devices Database is not merely an asset; it is an essential skill for researchers engaged in medical device innovation and regulatory compliance. This comprehensive guide elucidates the significance of the database, detailing its various components and the pivotal role it plays in facilitating informed decision-making and enhancing research outcomes. By adeptly navigating this resource, researchers can ensure compliance while leveraging invaluable insights that propel innovation in the MedTech sector.
Key arguments discussed throughout the article underscore the necessity of understanding the structure of the FDA Devices Database, accessing it correctly, utilizing its search features, interpreting device data, and remaining informed about updates. Each step outlined—from visiting the FDA website to employing advanced search techniques—equips researchers with the essential tools needed to efficiently gather and analyze critical information. Furthermore, the discourse on regulatory classifications and approval processes emphasizes the imperative of a thorough understanding of compliance requirements, ultimately fostering the development of safer and more effective medical devices.
In conclusion, the FDA Devices Database stands as a cornerstone for researchers striving to navigate the complexities of medical device regulation and development. By actively engaging with the database and employing the strategies outlined, researchers can deepen their understanding of regulatory frameworks, ensuring compliance while contributing to advancements in patient safety and device efficacy. Embracing this knowledge not only empowers individual research efforts but also fortifies the overall integrity of the medical device industry.
What is the FDA Devices Database?
The FDA Devices Database is a vital resource that catalogs medical instruments authorized for use in the United States. It includes several key databases such as the 510(k) Premarket Notification Database, the Premarket Approval (PMA) Database, and the Global Unique Device Identification Database (GUDID).
Why is the FDA Devices Database important for researchers?
The FDA Devices Database is important for researchers as it helps them monitor classifications, approvals, and post-market surveillance of medical devices. It also empowers them to efficiently locate and interpret device information relevant to their studies, ultimately enhancing research outcomes and driving innovation in the MedTech field.
How can one access the FDA Devices Database?
To access the FDA Devices Database, visit the official FDA website at www.fda.gov, click on the 'Medical Devices' tab, select 'Medical Device Databases' under the 'Device Advice' section, and choose the appropriate resource. Some databases may require account creation for full access.
What types of services does bioaccess® provide for clinical trials?
Bioaccess® offers comprehensive clinical trial management services, including feasibility studies, site selection, compliance reviews, trial setup, import permits, project management, and reporting.
What experience does bioaccess® have in clinical trials?
Bioaccess® has over 20 years of experience overseeing Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies (PMCF), equipping researchers with essential tools and knowledge to navigate clinical trials and regulatory environments effectively.
What is the significance of the 510(k) database?
The 510(k) database is frequently utilized for its comprehensive information on device clearances, making it a key resource for researchers and industry professionals navigating the FDA Devices Database.
How does understanding the FDA Devices Database contribute to compliance?
Mastering the FDA Devices Database is essential for ensuring adherence to evolving regulatory frameworks, as compliance is viewed as a continuous commitment to patient safety and product quality, rather than just a hurdle to overcome.