


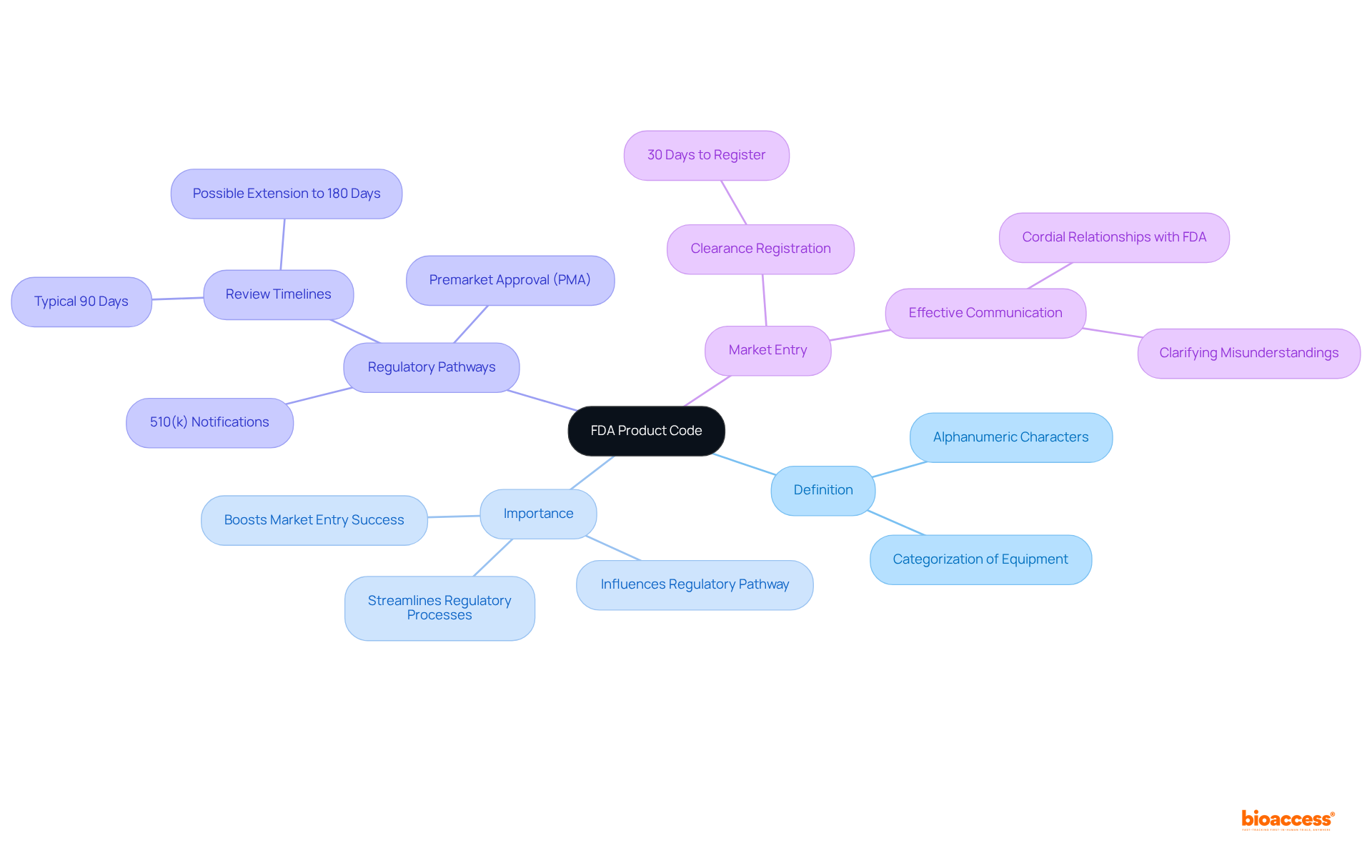
The FDA product code serves as a vital identifier for medical devices, categorizing them according to their intended use and technological characteristics. This categorization directly influences the regulatory pathway for market entry. Mastering this code not only streamlines the regulatory process but also significantly enhances the likelihood of successful approvals. By aligning with specific premarket filing requirements, it facilitates effective communication with the FDA, making it an essential aspect of navigating the Medtech landscape.
Understanding the intricacies of the FDA product code is essential for any Medtech innovator navigating the complex regulatory landscape of medical devices. This unique identifier categorizes equipment based on its intended use and technological attributes, playing a pivotal role in determining the regulatory pathway for approval. With high stakes and fierce competition, how can companies effectively leverage this critical tool to streamline their submission process and enhance their chances of market success?
The FDA product code functions as a unique identifier for medical instruments, consisting of five to seven alphanumeric characters that categorize equipment based on their intended purpose and technological attributes. This program is essential for Medtech innovators, as it directly influences the regulatory pathway a device must traverse. Understanding the product identifier is vital for recognizing specific premarket filing requirements, such as 510(k) notifications and Premarket Approval (PMA). Mastery of the product identifier not only streamlines regulatory processes but also significantly boosts the chances of successful market entry.
For instance, organizations that effectively utilize the product identifier can reduce the time to approval. The FDA typically evaluates 510(k) applications within 90 calendar days; however, the review period can often extend to six to nine months, and possibly up to 180 days if additional information is necessary. Furthermore, a thorough understanding of the product specifications can lead to better alignment with FDA expectations. Companies that maintain clear and respectful communication during the review process are more likely to achieve favorable outcomes.
Recent updates in the FDA's approach to product classifications underscore the importance of staying informed about regulatory changes, which can further influence the success of medical equipment submissions. By adeptly leveraging the FDA product code, Medtech companies can navigate the intricate regulatory pathways and position themselves for success in a competitive market.
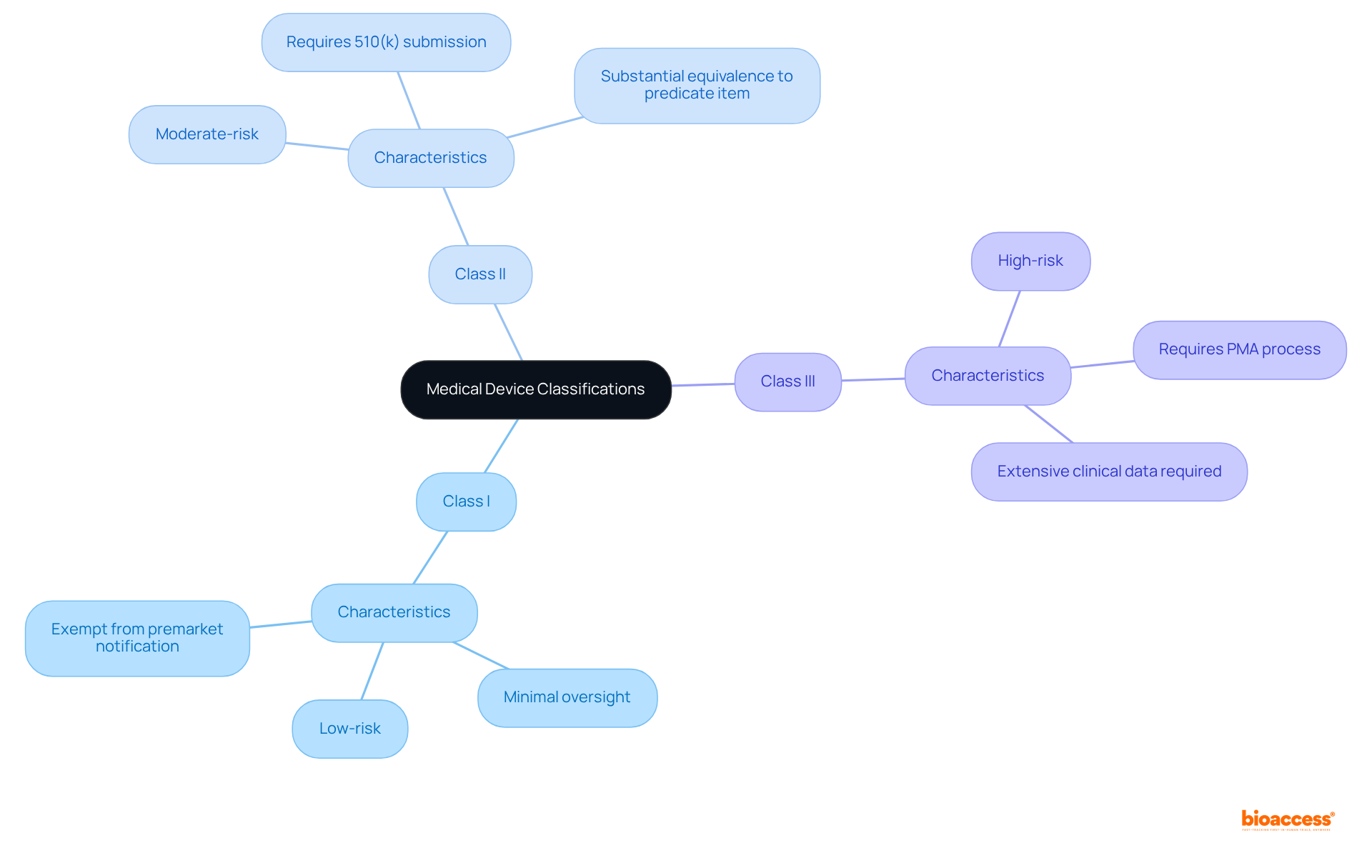
The FDA categorizes medical instruments into three primary groups:
Understanding the FDA product code classifications is crucial for Medtech companies, as it dictates the regulatory pathway and the type of evidence required for approval.
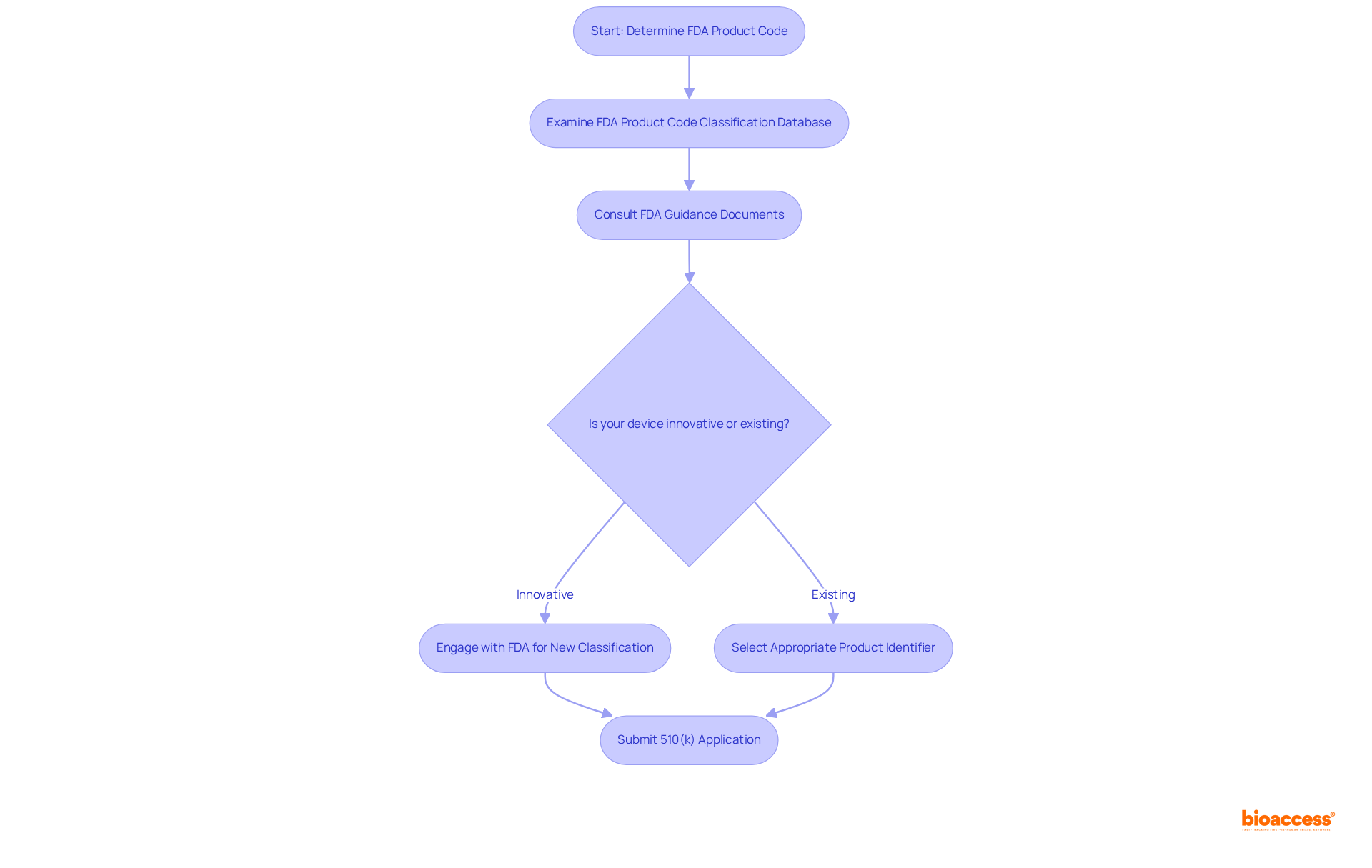
To determine the appropriate FDA product code for your medical equipment, it is essential to start by examining the FDA's Product Code Classification Database. This essential tool enables users to look up current product identifiers based on specific equipment traits and intended applications. Utilizing this database is crucial, as nearly two-thirds of 510(k) filings experience delays due to Additional Information (AI) requests, which often stem from insufficient product descriptions or misalignment with the selected product identifier. Notably, approximately 30% of entries are initially rejected for being incomplete, highlighting the necessity for meticulous documentation.
Consulting the FDA's guidance documents and classification panels can further illuminate the regulatory landscape. It is vital to ensure that the chosen product identifier accurately reflects your equipment's specifications and intended use, as this alignment directly influences the regulatory requirements and application process. Should your device be innovative or not conform to existing codes, it may be necessary to engage with the FDA to pursue a new classification, which can be initiated through a 513(g) request for information. The FDA is obligated to respond to these requests within 60 days, providing a clear timeline for your classification process.
Regular updates to the database of FDA product codes enhance its utility, allowing manufacturers to remain informed about the latest classifications and requirements. By effectively utilizing this resource, companies can streamline their application processes and improve their chances of achieving favorable regulatory outcomes. As emphasized by FDA officials, the FDA product code is a critical component of the Product Code Classification Database, which serves as an essential resource for ensuring compliance and facilitating the approval process. Furthermore, it is important to note that the typical 510(k) application encompasses around 1,200 pages, underscoring the complexity and thoroughness required in the process.
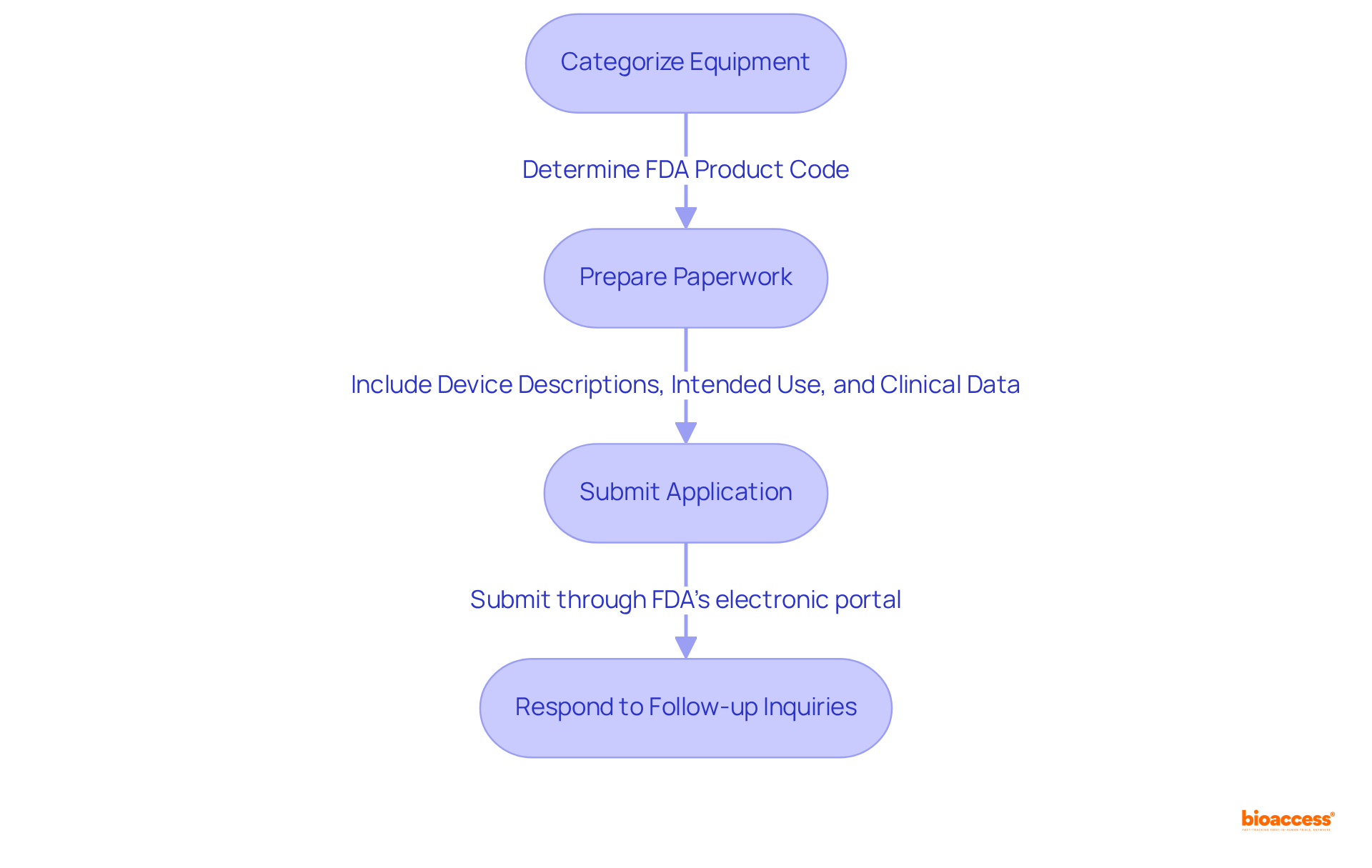
Navigating the FDA application process necessitates a systematic approach. Begin by categorizing your equipment to determine the appropriate FDA product code, which is essential for establishing the entry route. The next phase involves preparing the necessary paperwork, which may include a 510(k) filing or a PMA application, depending on the product's classification. Key documents typically consist of:
Once your entry is finalized, it should be submitted through the FDA's electronic portal. Be prepared for follow-up inquiries from the FDA, as 67 percent of submissions lead to requests for additional information during the substantive review process. This proactive strategy can significantly streamline the approval timeline, which averages around 147 days, although the FDA strives for a 90-day decision target. Understanding these steps and maintaining thorough documentation will facilitate a smoother path to market entry.
Mastering the FDA product code is essential for companies in the Medtech industry aiming for regulatory success. This unique identifier categorizes medical devices and significantly impacts the approval process, influencing everything from premarket filing requirements to the overall timeline for market entry. By understanding and effectively utilizing the product code, innovators can navigate the complex regulatory landscape more efficiently, positioning their devices for success.
The article explored key aspects such as:
The distinctions between Class I, II, and III devices highlight the varying levels of regulatory oversight and the importance of accurately reflecting a device's intended use in the product code selection. Furthermore, the emphasis on thorough documentation and proactive communication with the FDA is crucial, as these practices are essential for minimizing delays and achieving favorable outcomes.
In conclusion, the significance of the FDA product code extends beyond mere categorization; it is a foundational element that shapes the entire regulatory pathway for medical devices. Companies are encouraged to leverage available resources, such as the Product Code Classification Database, to enhance their understanding and ensure compliance. By investing the necessary time and effort into mastering the intricacies of the FDA product code and submission process, Medtech innovators can streamline their path to market and contribute to the advancement of healthcare solutions that benefit patients worldwide.
What is the FDA product code?
The FDA product code is a unique identifier for medical instruments, consisting of five to seven alphanumeric characters that categorize equipment based on their intended purpose and technological attributes.
Why is the FDA product code important for Medtech innovators?
The FDA product code is crucial for Medtech innovators as it directly influences the regulatory pathway a device must follow and helps in recognizing specific premarket filing requirements, such as 510(k) notifications and Premarket Approval (PMA).
How does understanding the FDA product code benefit companies?
Mastery of the FDA product code can streamline regulatory processes, reduce time to approval, and significantly increase the chances of successful market entry for medical devices.
What is the typical review time for 510(k) applications?
The FDA typically evaluates 510(k) applications within 90 calendar days, but the review period can extend to six to nine months, and possibly up to 180 days if additional information is required.
How can effective communication during the review process impact outcomes?
Companies that maintain clear and respectful communication during the review process are more likely to achieve favorable outcomes with their submissions.
Why is it essential to stay informed about regulatory changes?
Staying informed about regulatory changes is essential because recent updates in the FDA's approach to product classifications can influence the success of medical equipment submissions.
How can Medtech companies leverage the FDA product code for success?
By adeptly leveraging the FDA product code, Medtech companies can navigate complex regulatory pathways and position themselves effectively in a competitive market.