


The article titled "Master the INVIMA Fee Calculator 2025: Step-by-Step Guide" serves as an essential resource for effectively utilizing the INVIMA fee calculator, enabling users to accurately estimate costs associated with the registration of medical devices and pharmaceuticals in Colombia. It underscores the critical significance of cost transparency and regulatory compliance within the industry.
Detailed instructions and troubleshooting tips are provided, ensuring users can navigate the calculator with confidence. This guidance not only facilitates a smoother enrollment process but also enhances overall market entry efficiency, addressing key challenges faced by stakeholders in the Medtech landscape.
Understanding the financial landscape of medical device and pharmaceutical registration in Colombia is crucial for stakeholders aiming to thrive in a competitive market. The INVIMA fee calculator for 2025 emerges as a vital tool, offering clarity and precision in cost estimation, significantly streamlining the registration process.
However, navigating this calculator effectively poses its own set of challenges—what information is essential, and how can users avoid common pitfalls? This guide delves into the intricacies of the INVIMA fee calculator, ensuring that users are well-equipped to tackle the complexities of regulatory fees and enhance their market entry strategies.
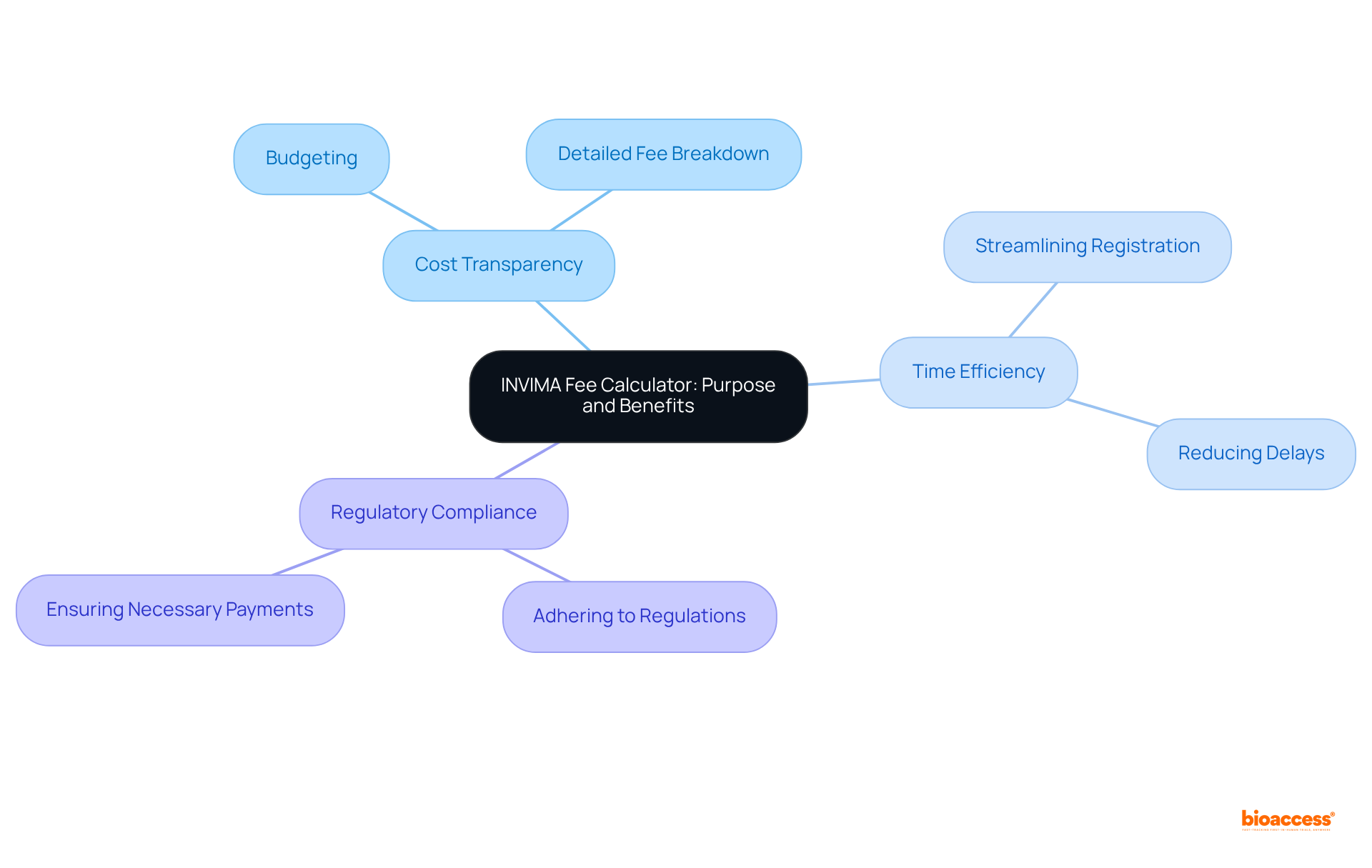
The invima fee calculator 2025 serves as an essential tool for calculating costs associated with the approval of medical devices and pharmaceuticals in Colombia. By providing a transparent breakdown of expenses, it empowers stakeholders to budget effectively and mitigate unforeseen costs. The benefits of utilizing this calculator are significant:
The regulatory authority offers various services related to medical instruments, including monitoring and controlling safety and effectiveness, which are crucial for successful market entry. In a market where the medical equipment sector is projected to reach a volume of USD 3.44 billion by 2030, the importance of cost transparency cannot be overstated. Companies that leverage tools such as the invima fee calculator 2025 are better positioned to navigate the complexities of registration, ultimately facilitating faster market entry and enhancing their competitive edge. This proactive approach to financial planning is essential, particularly as the demand for innovative medical products continues to rise, influenced by factors such as an aging population and increasing healthcare needs in Colombia.
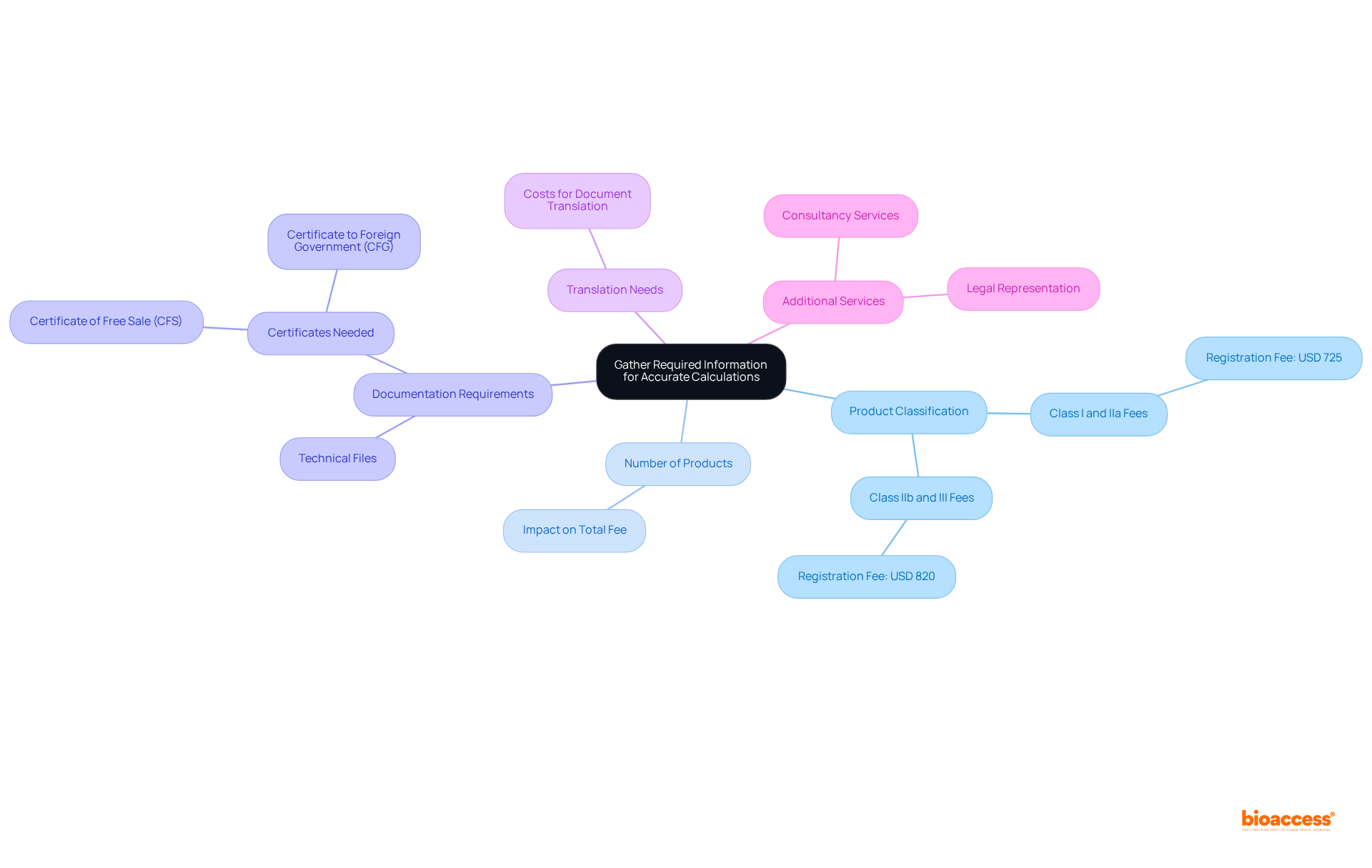
To effectively utilize the invima fee calculator 2025, it is essential to gather specific information that will influence the fee structure. Here’s what to collect:
Product Classification: Determine the categorization of your medical apparatus or pharmaceutical item, as charges vary according to risk categories. For instance, registration fees are USD 725 for Class I and IIa categories, while Class IIb and III categories incur fees of USD 820. Comprehending these expenses is vital for financial planning, particularly considering INVIMA's responsibility in guaranteeing the safety, efficacy, and quality of medical products as a Level 4 health authority acknowledged by PAHO/WHO.
Number of Products: Be aware of how many products you are registering, as this can affect the total fee. The more products you register, the more comprehensive your fee estimation will be.
Documentation Requirements: Familiarize yourself with the necessary documentation, including certificates of free sale and technical files. A Certificate of Free Sale (CFS) or Certificate to Foreign Government (CFG) is required for importing medical devices into South America. These documents are essential for the enrollment procedure and may incur additional costs if not prepared correctly.
Translation Needs: If any documents require translation into Spanish, consider those costs as well. Accurate translations are vital for compliance with INVIMA regulations, which oversee the marketing and manufacturing of health products in Colombia.
Additional Services: Evaluate any extra services you might need, such as legal representation or consultancy. These services can increase the total costs but may also simplify the enrollment process.
Having this information prepared will aid in your use of the invima fee calculator 2025 and ensure that you obtain a precise estimate of the costs involved. Comprehending how product classification influences regulatory fees and the overall registration process is crucial for successful market entry.
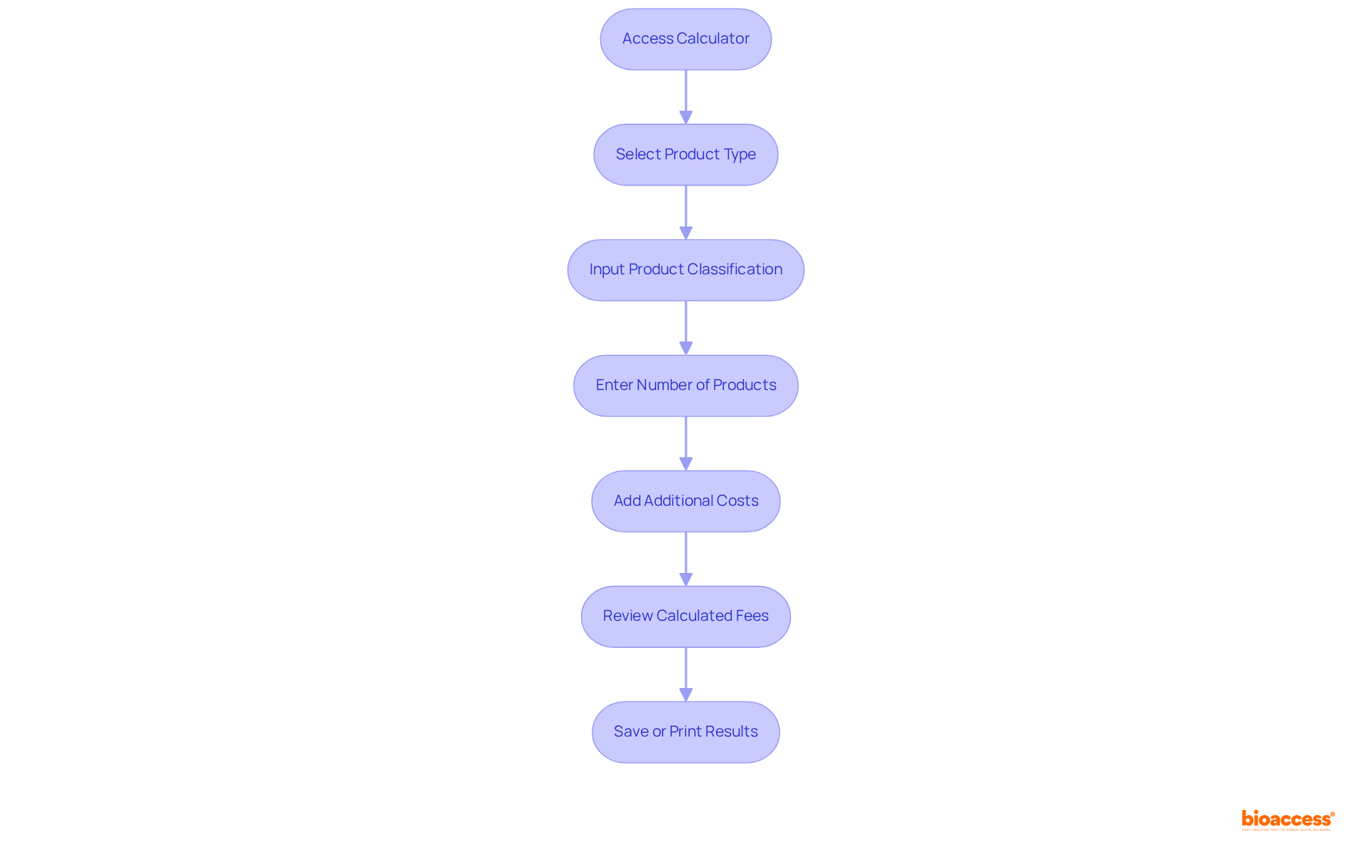
To effectively navigate the INVIMA Fee Calculator, follow these detailed steps:
Utilizing the invima fee calculator 2025 can greatly simplify your enrollment process. It is essential to submit a complete application to ensure a smooth review process by the agency, which is recognized as a Level 4 health authority by PAHO/WHO, underscoring its competence in regulating health products.
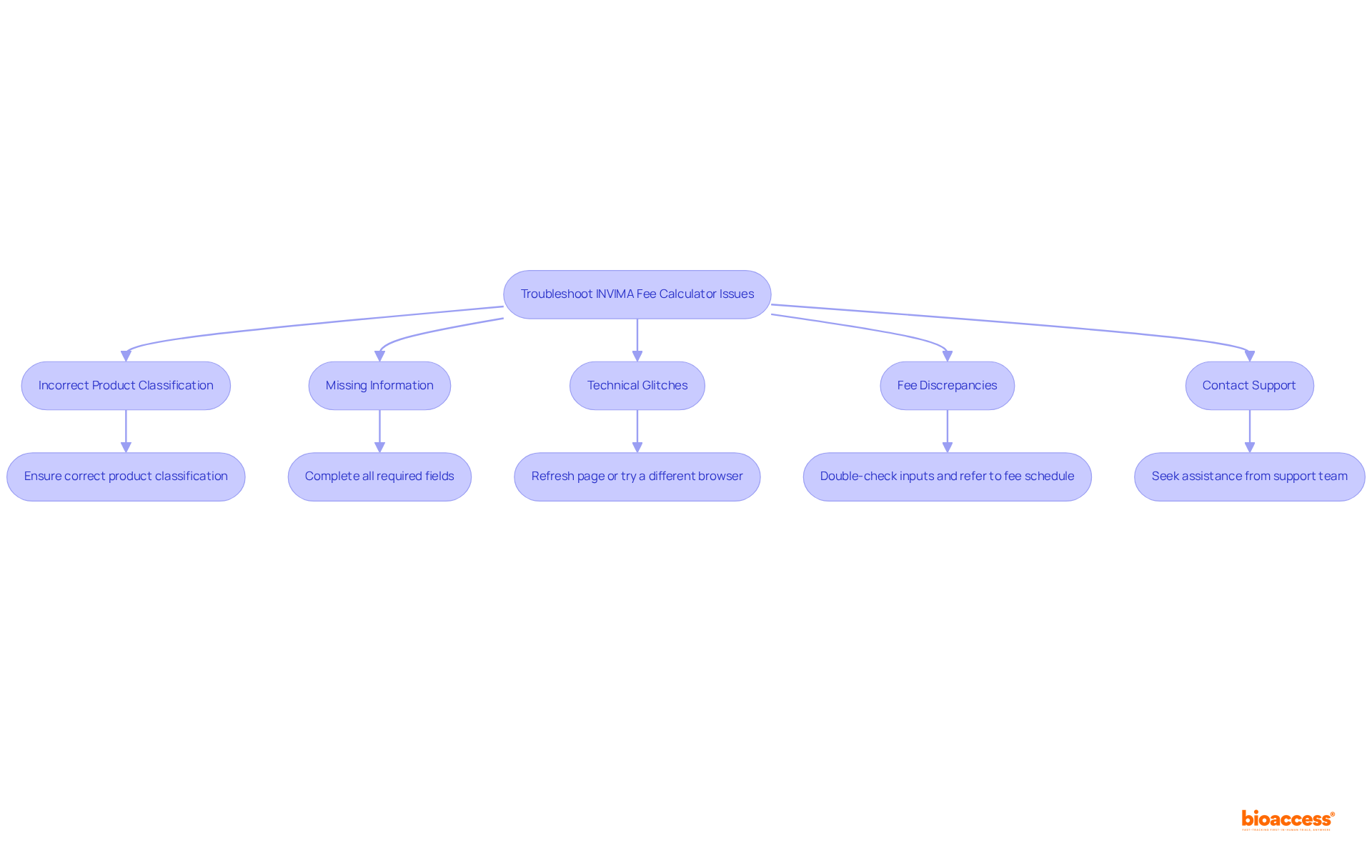
When utilizing the invima fee calculator 2025, several frequent problems may obstruct your enrollment process. Here’s how to troubleshoot them effectively:
Incorrect Product Classification: Ensure that you have selected the appropriate product classification. Misclassifications can lead to flawed fee estimates, complicating your enrollment. Precise categorization is essential, as it directly influences the approval schedule and overall market entry plan, reflecting the strict regulatory oversight of medical devices.
Missing Information: Verify that all required fields are completed. Omitting necessary information can prevent the calculator from functioning correctly, leading to delays in your registration process.
Technical Glitches: If the calculator becomes unresponsive or displays errors, refresh the page or try accessing it from a different browser to resolve potential technical issues. Technical difficulties can be common, especially during peak usage times.
Fee Discrepancies: If the calculated fees do not match your expectations, double-check your inputs for accuracy and refer to the fee schedule for confirmation. Understanding the fee structure is essential.
Contact Support: Should issues continue, reach out to the support team for assistance. They can provide guidance and help resolve any technical difficulties you may encounter.
By understanding these common issues and their solutions, you can navigate the invima fee calculator 2025 more efficiently, ensuring a smoother registration process. Furthermore, learning from firms that have effectively addressed product classification mistakes can offer valuable insights into best practices, emphasizing the significance of adhering to regulatory standards. Notably, INVIMA is classified as a Level 4 health authority by PAHO/WHO, underscoring its significant role in ensuring the safety and efficacy of medical devices.
Mastering the INVIMA fee calculator for 2025 is essential for stakeholders engaged in the registration of medical devices and pharmaceuticals in Colombia. This tool not only facilitates accurate financial planning but also ensures compliance with regulatory requirements, ultimately supporting a smoother market entry process.
The article underscores the key benefits of utilizing the INVIMA fee calculator, encompassing cost transparency, time efficiency, and the critical importance of gathering accurate information for precise calculations. By comprehending product classifications, potential additional costs, and common troubleshooting techniques, users can navigate the registration process with confidence and clarity.
In conclusion, leveraging the INVIMA fee calculator stands as a vital step for businesses striving to excel in Colombia's expanding medical equipment sector. By proactively addressing potential challenges and utilizing the resources available, stakeholders can enhance their competitive edge and ensure successful compliance with INVIMA regulations. Embrace this tool to streamline your registration efforts and position your products for success in the marketplace.
What is the purpose of the INVIMA Fee Calculator?
The INVIMA Fee Calculator serves to calculate costs associated with the approval of medical devices and pharmaceuticals in Colombia, providing a transparent breakdown of expenses to help stakeholders budget effectively.
What are the benefits of using the INVIMA Fee Calculator?
The benefits include cost transparency, time efficiency, and regulatory compliance. It allows users to accurately plan finances, streamline the registration process, and ensure all necessary payments are made to adhere to regulations.
How does the INVIMA Fee Calculator promote cost transparency?
The calculator provides a detailed fee breakdown, enabling users to be aware of all potential costs involved in the registration process, which helps in accurate financial planning.
In what way does using the calculator improve time efficiency?
By calculating fees in advance, users can reduce potential delays in the registration process that may arise from financial uncertainties.
Why is regulatory compliance important when using the INVIMA Fee Calculator?
A clear understanding of the fee structure ensures that all necessary payments are made, which is vital for maintaining adherence to regulations.
What is the projected market volume for the medical equipment sector in Colombia by 2030?
The medical equipment sector in Colombia is projected to reach a volume of USD 3.44 billion by 2030.
How does the INVIMA Fee Calculator help companies in the medical device sector?
It helps companies navigate the complexities of registration, facilitating faster market entry and enhancing their competitive edge in a growing market influenced by increasing healthcare needs.