


This article provides a comprehensive overview of the medical device registration timeline in Mexico, outlining the essential steps and requirements for compliance with COFEPRIS regulations. Understanding device classification is crucial, as is the appointment of a local representative and the maintenance of thorough documentation. These elements are vital for navigating the registration process effectively, ultimately ensuring timely market entry for medical devices. By grasping these key aspects, stakeholders can better position themselves within the Medtech landscape, addressing the challenges that arise in clinical research.
Navigating the medical device registration landscape in Mexico presents a significant challenge, particularly in light of the evolving regulations established by COFEPRIS. With the market for medical equipment projected to soar to an impressive $12.6 billion by 2029, it is essential to grasp the complexities of the registration timeline and requirements for successful market entry. Given the varying classifications and unique documentation demands, how can manufacturers facilitate a smooth and efficient approval process? This guide explores the fundamental steps and strategies necessary to master the medical device registration timeline in Mexico, equipping stakeholders to navigate challenges and capitalize on opportunities within this competitive environment.
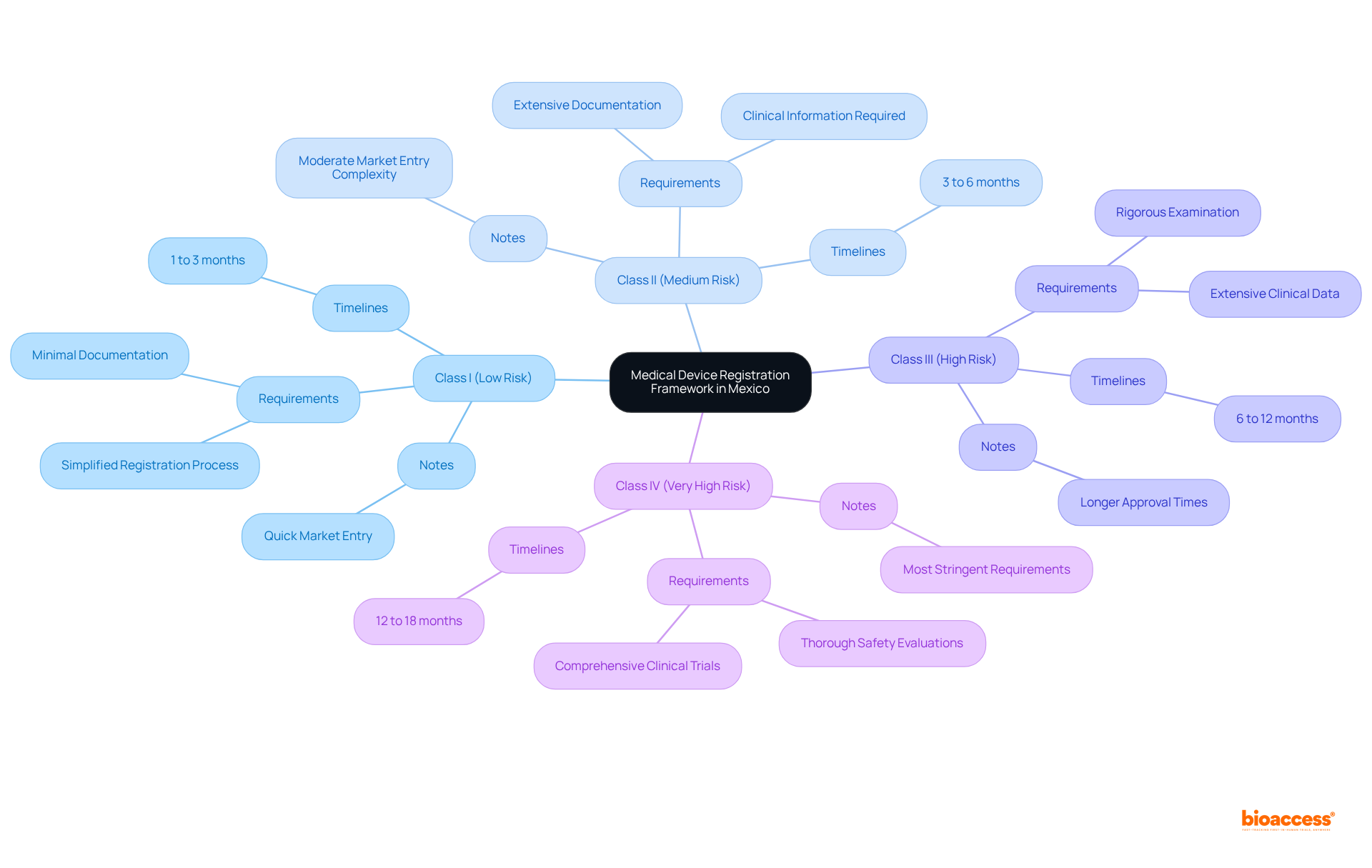
The medical equipment approval procedure in Mexico is under the supervision of the Federal Commission for Protection against Sanitary Risks (COFEPRIS). This regulatory framework categorizes medical equipment into four distinct classes based on risk levels:
Each class presents unique requirements and timelines for enrollment, with Class I devices typically facing the least stringent documentation demands, thereby facilitating quicker market entry in the context of the medical device registration timeline Mexico. Understanding the General Health Law and specific guidelines from the health regulatory agency is crucial for ensuring compliance. Furthermore, Good Manufacturing Practices (GMP) are integral to the approval process, and appointing a local representative is mandatory for effectively navigating the regulatory landscape.
Recent updates to COFEPRIS regulations in 2025 highlight ongoing adaptations to align with advancements in medical technology, underscoring the necessity of staying informed about these changes. Knowledge of classification statistics and approval systems significantly enhances the prospects of successful market entry for medical products in Mexico, especially when considering the medical device registration timeline Mexico. Notably, the medical equipment market in Mexico is projected to grow at a 7.6% CAGR from 2024 to 2029, reaching a market value of $12.6 billion, which emphasizes the importance of comprehending the registration process.
Moreover, it is essential to acknowledge that 90% of medical equipment in Mexico is imported, illustrating the competitive environment for foreign producers. Leveraging the expertise of professionals such as Ana Criado, who possesses extensive experience in regulatory affairs, alongside the comprehensive clinical trial management services provided by bioaccess®, including feasibility studies, site selection, and compliance reviews, can offer invaluable support in navigating this complex regulatory landscape.
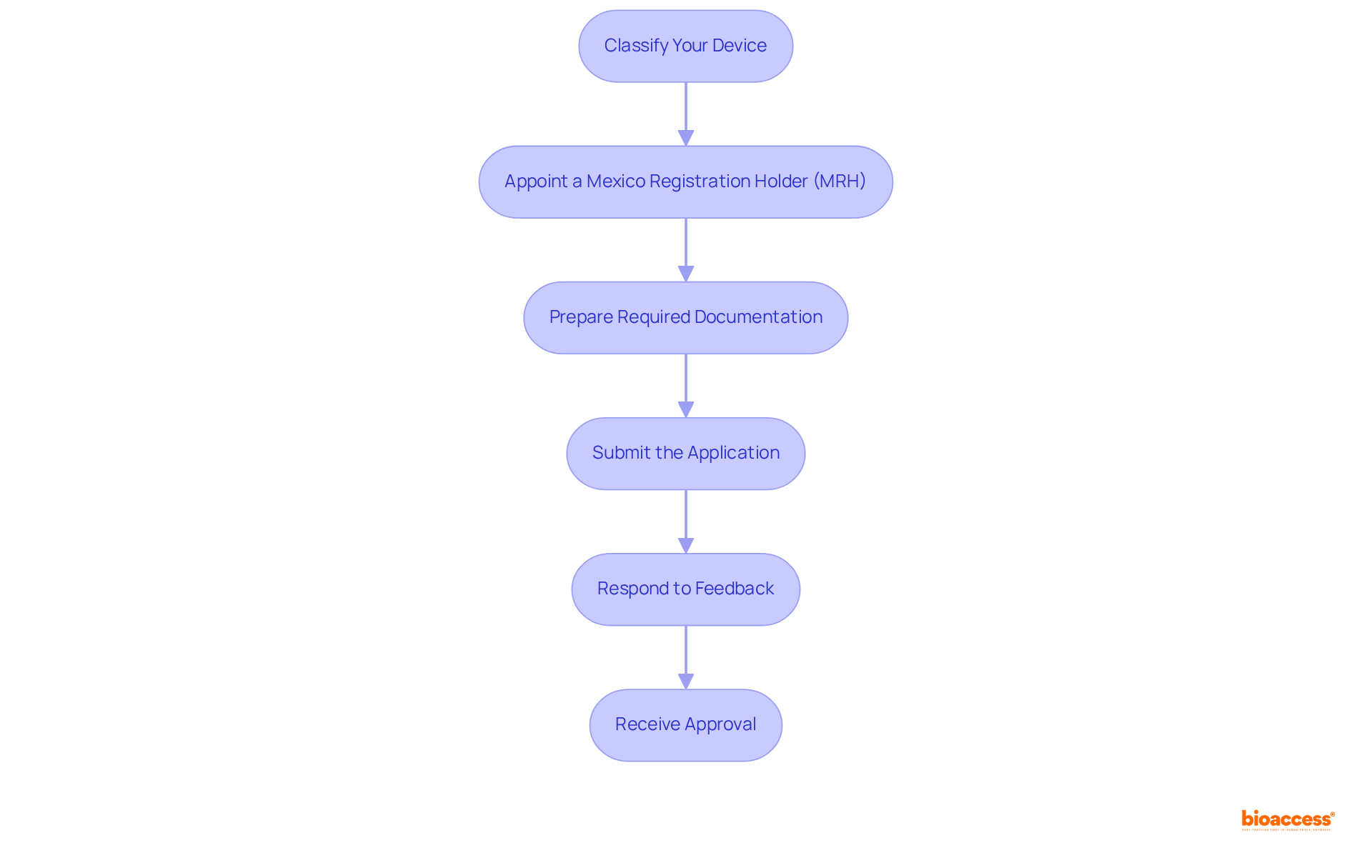
Classify Your Device: Begin by determining the classification of your medical device according to regulatory guidelines. This classification is essential as it dictates the specific enrollment criteria and the anticipated medical device registration timeline in Mexico.
Appoint a Mexico Registration Holder (MRH): For foreign manufacturers, appointing a local representative is critical. The MRH will oversee the enrollment system and serve as the primary contact with the health authority, ensuring compliance with local regulations.
Prepare Required Documentation: Compile all necessary documents, including technical specifications, proof of Good Manufacturing Practices (GMP) compliance, and labeling requirements in Spanish. Examine all documents meticulously for thoroughness and correctness to prevent delays in the medical device registration timeline Mexico.
Submit the Application: File your registration application with the relevant authority, ensuring that all required documentation is included. Be prepared to pay any associated fees, which may vary based on the device classification.
Respond to Feedback: Following submission, the regulatory agency may request additional information or clarifications. Timely responses to these inquiries are essential for maintaining momentum in the medical device registration timeline Mexico approval process.
Receive Approval: Upon approval, COFEPRIS will issue a certification, permitting you to market your device in Mexico. Familiarize yourself with the validity period of the enrollment and the renewal requirements to ensure ongoing compliance.
In this context, leveraging the expertise of professionals like Ana Criado, who possesses extensive experience in regulatory affairs and market access strategies in Latin America, can provide invaluable insights into overcoming challenges and seizing opportunities within the Medtech landscape.
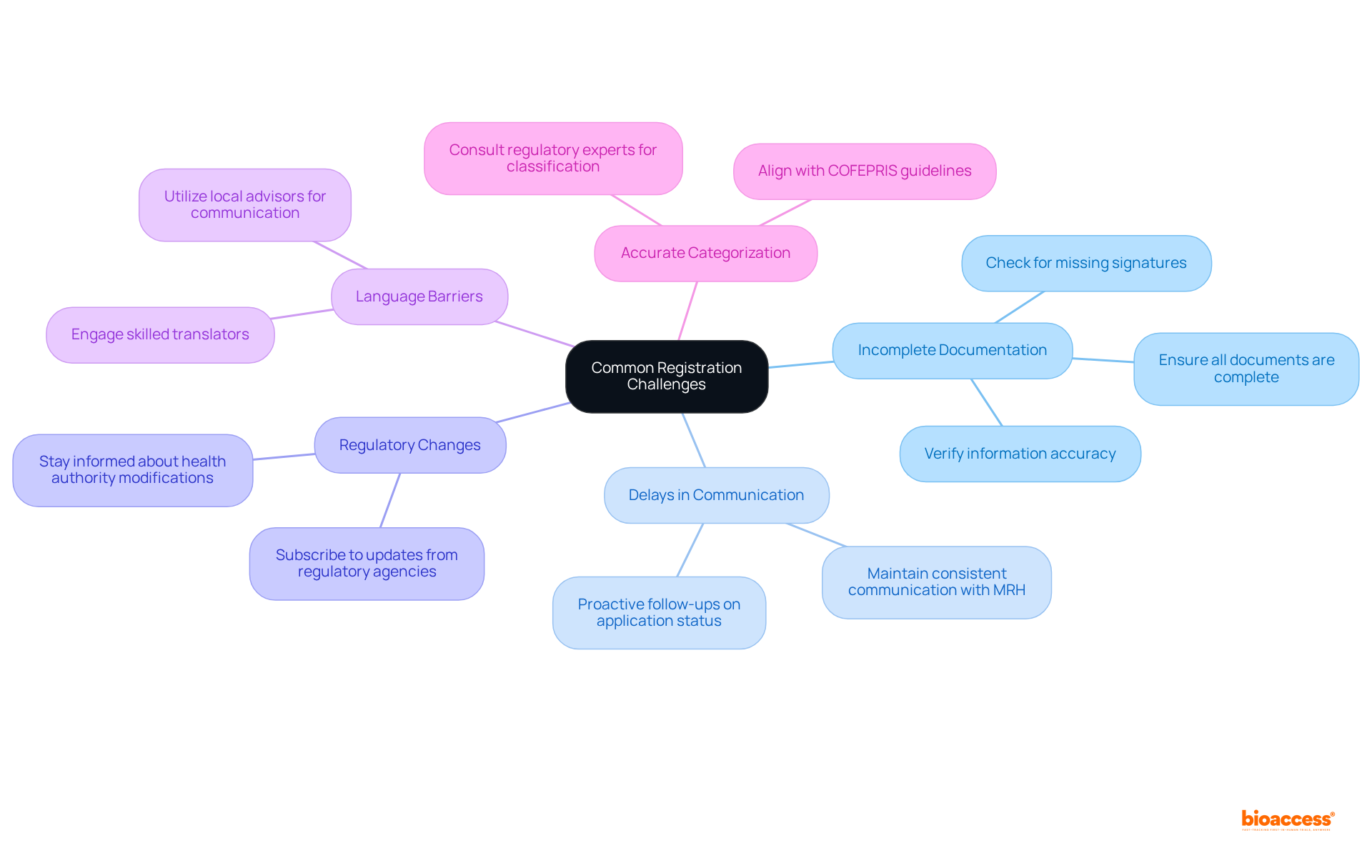
Incomplete Documentation: Ensuring that all required documents are complete and formatted correctly is crucial. Missing signatures or incorrect information can lead to significant delays in the medical device registration timeline Mexico. By consistently examining documentation against regulatory requirements, you can effectively reduce these issues.
Delays in Communication: It is essential to maintain consistent communication with your Mexican Registration Holder (MRH) and the relevant authorities to stay updated on your application status. In the event of delays regarding the medical device registration timeline Mexico, proactive follow-ups can clarify the reasons behind them and expedite the process.
Regulatory changes regarding the medical device registration timeline in Mexico are constantly evolving, making it essential to stay informed about modifications in health authority regulations. Subscribing to updates from relevant regulatory agencies or industry groups can provide timely information that may significantly influence your application.
Language Barriers: Navigating the registration process can pose challenges if you are not fluent in Spanish. Engaging a skilled translator or local advisor can enhance the accuracy of documentation and facilitate efficient communication with the regulatory body, particularly concerning the medical device registration timeline in Mexico, thereby minimizing the risk of misunderstandings.
Accurate categorization of your medical apparatus is vital to adhere to the medical device registration timeline in Mexico. Misclassification can lead to inappropriate requirements and unnecessary delays. Consulting with regulatory experts ensures that your product is classified correctly, in alignment with COFEPRIS guidelines.
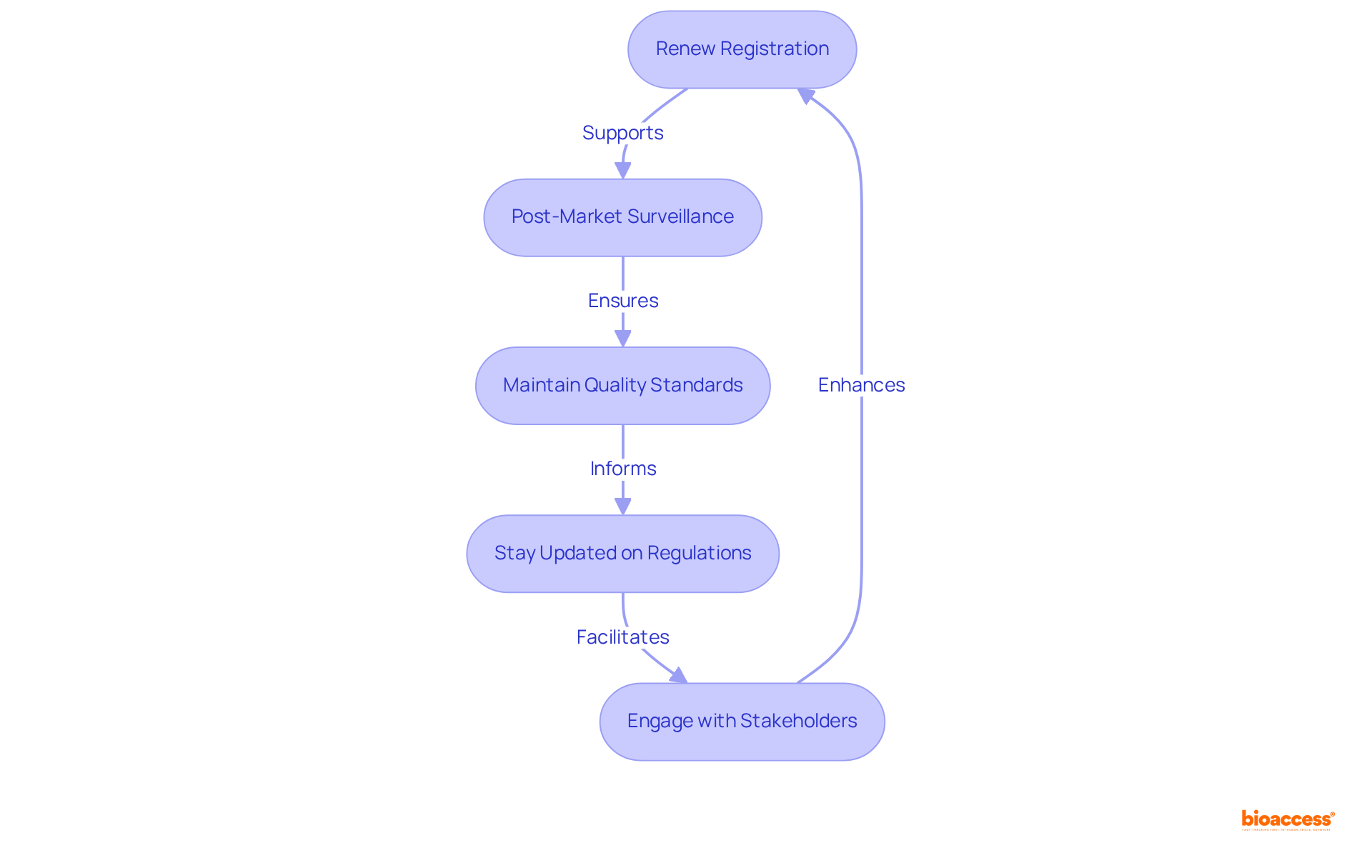
Renew Registration: In Mexico, the medical device registration timeline indicates that medical equipment registrations are valid for five years, making proactive renewal management essential. To prevent compliance lapses, begin the renewal process at least 150 days prior to expiration, as the medical device registration timeline indicates that this initial extension phase tends to be the most labor-intensive. The option to submit renewals electronically now streamlines this process significantly.
Post-Market Surveillance: A robust post-market surveillance system is vital for monitoring your product's performance. This includes tracking negative occurrences and ensuring timely reporting to the regulatory authority, which is crucial for maintaining compliance and safeguarding patient safety. Effective systems are indispensable for early identification of potential issues and ensuring regulatory adherence.
Maintain Quality Standards: Continuous compliance with Good Manufacturing Practices (GMP) and other quality standards is imperative. Regular audits and inspections are necessary to uphold your registration status and confirm that your equipment meets the required safety and efficacy standards.
Stay Updated on Regulations: It is essential to stay informed about COFEPRIS updates and regulatory changes that may affect your device. Recent updates, effective July 10, 2023, underscore the necessity of adapting compliance strategies to align with new requirements, including the medical device registration timeline, as well as documentation and submission processes.
Engage with Stakeholders: Cultivating open communication with your Mexico Registration Holder (MRH), healthcare professionals, and regulatory bodies is vital. This collaboration is key to effectively meeting compliance requirements and addressing emerging challenges in the regulatory landscape. Additionally, utilizing comprehensive clinical trial management services, such as those provided by bioaccess, can facilitate the feasibility studies, site selection, compliance reviews, trial setup, import permits, project management, and reporting necessary for successful market entry in Mexico.
Navigating the medical device registration timeline in Mexico represents a complex yet essential undertaking for manufacturers aspiring to penetrate this burgeoning market. Understanding the regulatory framework established by COFEPRIS, alongside the specific requirements for each device classification, enables stakeholders to streamline their approach and significantly enhance their chances of successful market entry. The necessity of appointing a local representative and adhering to Good Manufacturing Practices cannot be overstated; these elements are critical for ensuring compliance and facilitating a smoother registration journey.
This article outlines key steps in the registration process, including:
It also addresses common challenges such as:
Offering valuable insights into effectively navigating these hurdles. By staying informed and engaging with regulatory experts, manufacturers position themselves to overcome obstacles and achieve timely approvals.
Ultimately, grasping the medical device registration timeline in Mexico transcends mere compliance; it embodies the pursuit of opportunities within a competitive market projected for significant growth in the coming years. By prioritizing thorough preparation, maintaining open communication with regulatory bodies, and ensuring ongoing compliance post-registration, manufacturers can safeguard their investments and contribute to the advancement of healthcare in Mexico. Embracing these strategies will undoubtedly pave the way for success in the dynamic landscape of medical device regulation.
What is the regulatory body overseeing medical device registration in Mexico?
The regulatory body overseeing medical device registration in Mexico is the Federal Commission for Protection against Sanitary Risks (COFEPRIS).
How are medical devices classified in Mexico?
Medical devices in Mexico are classified into four distinct classes based on risk levels: Class I (low risk), Class II (medium risk), Class III (high risk), and Class IV (very high risk).
What are the requirements for Class I medical devices?
Class I medical devices typically face the least stringent documentation demands, which facilitates quicker market entry compared to higher-risk classes.
Why is it important to understand the General Health Law and specific guidelines from COFEPRIS?
Understanding the General Health Law and specific guidelines from COFEPRIS is crucial for ensuring compliance with the medical device registration process in Mexico.
What role do Good Manufacturing Practices (GMP) play in the approval process?
Good Manufacturing Practices (GMP) are integral to the approval process for medical devices, ensuring that products meet safety and quality standards.
Is it necessary to appoint a local representative for medical device registration in Mexico?
Yes, appointing a local representative is mandatory for effectively navigating the regulatory landscape in Mexico.
What recent updates have been made to COFEPRIS regulations?
Recent updates to COFEPRIS regulations in 2025 highlight ongoing adaptations to align with advancements in medical technology.
What is the projected growth rate of the medical equipment market in Mexico from 2024 to 2029?
The medical equipment market in Mexico is projected to grow at a 7.6% CAGR from 2024 to 2029, reaching a market value of $12.6 billion.
What percentage of medical equipment in Mexico is imported?
Approximately 90% of medical equipment in Mexico is imported.
How can professionals assist in navigating the medical device registration process?
Professionals with expertise in regulatory affairs, such as Ana Criado, and comprehensive clinical trial management services, like those provided by bioaccess®, can offer invaluable support in navigating the complex regulatory landscape.