


The article offers a comprehensive step-by-step guide to mastering the drug approval process. It details the roles of regulatory agencies, the phases of drug development, and the necessary documentation for successful application submissions. By emphasizing the importance of collaboration with stakeholders and adherence to regulatory requirements, it illustrates how these elements collectively contribute to the efficiency and success of bringing new medications to market.
Navigating the intricate landscape of drug approval is a formidable endeavor, particularly given the multitude of regulations and processes involved. As the demand for innovative therapies escalates, comprehending the steps necessary for successful medication authorization becomes increasingly crucial for developers and stakeholders alike.
What challenges may emerge in this complex journey, and how can one effectively prepare to surmount them? This guide explores the essential phases of drug development, regulatory requirements, and collaborative strategies designed to streamline the approval process and bolster the likelihood of introducing new treatments to market.
The medication authorization environment is primarily governed by oversight authorities, with the U.S. Food and Drug Administration (FDA) at the forefront. The FDA's responsibility is to ensure that new medications are safe and effective for public use, which is essential in the process of drug approval. Other regulatory bodies, such as the European Medicines Agency (EMA) and various national agencies, also make significant contributions within their jurisdictions. Familiarity with these organizations and their guidelines is essential for effectively navigating the endorsement process.
Understanding the different categories of medication authorizations is equally important. New Drug Applications (NDA) are mandatory for new molecular entities, while Abbreviated New Drug Applications (ANDA) cater to generics. Each category has specific requirements and timelines that must be adhered to during the process of drug approval for successful endorsement. In 2025, the FDA is expected to maintain a high acceptance rate, with projections indicating that approximately 40% of new medication authorizations will proceed through the 505(b)(2) pathway, which facilitates a more efficient process of drug approval by leveraging existing data.
Successful case studies underscore the FDA's role in medication authorizations. For instance, the endorsement of Spravato (esketamine) via the 505(b)(2) pathway exemplifies how existing medications can be repurposed to address new therapeutic needs. Similarly, Gleevec's authorization, despite not achieving its primary objective, highlights the FDA's willingness to evaluate additional evidence when assessing treatment effectiveness. These instances emphasize the importance of understanding regulatory expectations and the potential for innovative approaches in the process of drug approval and therapy development.
Expert insights highlight the dynamic nature of regulatory agencies. Recent discussions have revealed the FDA's increasing openness to real-world evidence, which could enhance the validation process for treatments targeting rare diseases. As the landscape continues to evolve, staying informed about the latest updates and trends in the process of drug approval is crucial for developers aiming to introduce new therapies to the market.

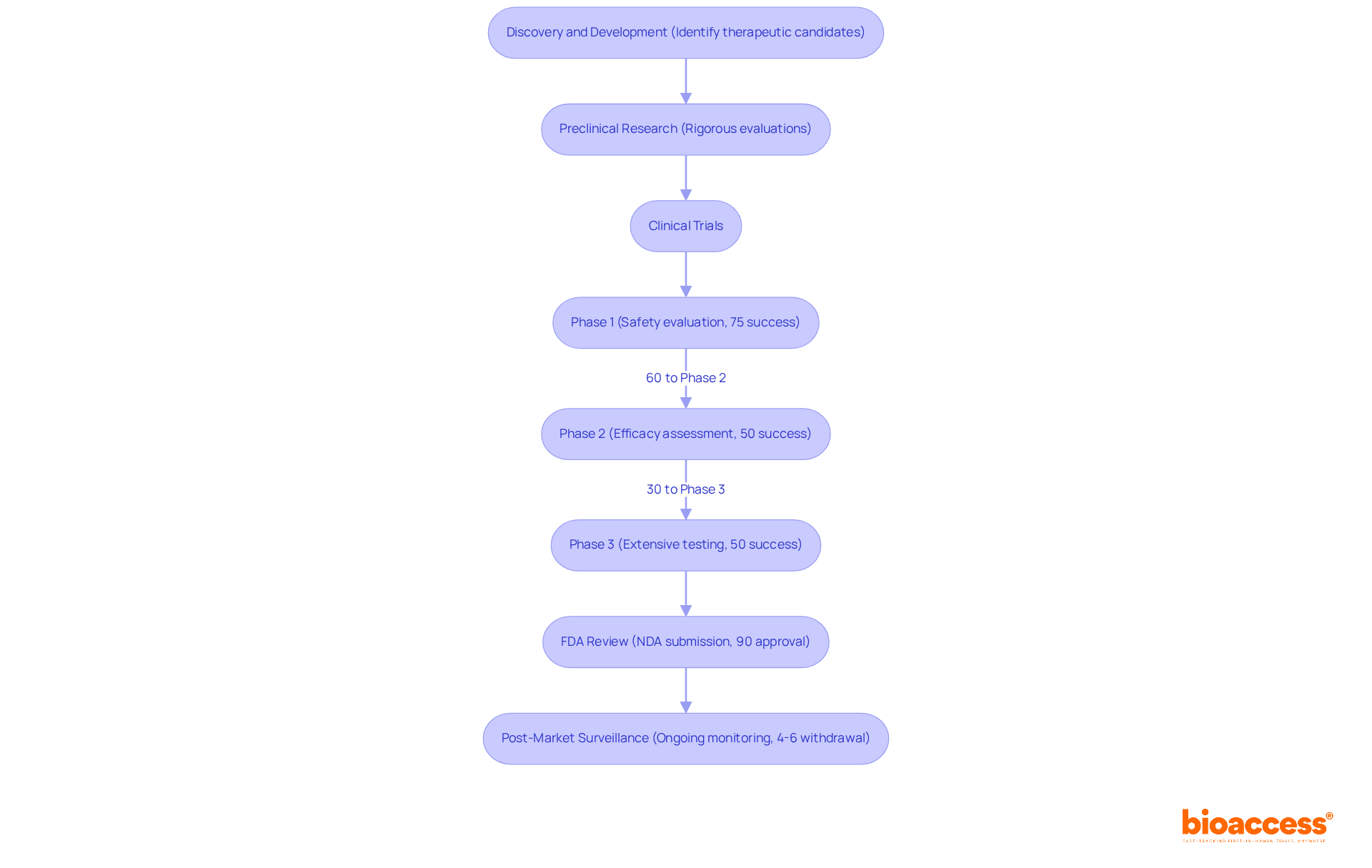
Drug development represents a structured process, meticulously divided into several critical phases that underscore its complexity and importance:
The discovery and development phase is pivotal in the process of drug approval, emphasizing the identification of potential therapeutic candidates through extensive research and development. Researchers conduct laboratory experiments to investigate the substance's mechanism of action and evaluate its potential effectiveness.
Preclinical Research: Prior to human trials, substances undergo rigorous preclinical evaluations in laboratory and animal studies to assess safety and biological activity. This phase is essential in the process of drug approval to determine whether a medication is safe enough to proceed to human trials.
Clinical Trials: This phase is segmented into three stages:
FDA Review: Following successful clinical trials, a New Drug Application (NDA) is submitted to the FDA for review. The process of drug approval can extend over several months to years, influenced by the substance's complexity and the strength of the data provided. Notably, the average time to introduce a medication to market exceeds 10 years, presenting significant challenges given the limited patent life of 20 years.
Post-Market Surveillance: Once a medication is approved and enters the market, ongoing monitoring is essential to track its long-term effects and ensure continued safety. Approximately 4%-6% of authorized medications may be removed from the market due to safety concerns that were not detected during clinical evaluations.
Recent trends indicate that sponsors are increasingly utilizing big data and innovative technologies to enhance clinical trial methods, aiming to shorten timelines and boost efficiency. Successful case studies highlight the significance of collaboration between academia and industry, resulting in improved therapeutic development outcomes, particularly in the field of biologics and vaccines, which exhibit greater success rates than conventional small molecules. Notably, bioaccess's accelerated regulatory approval process enables regulatory approval in just 6-8 weeks, compared to the typical 6-12 months in the US/EU, facilitating faster enrollment of treatment-naive cardiology or neurology cohorts. This efficiency not only expedites medication availability but also benefits local economies through job creation and healthcare enhancements. Furthermore, addressing patient recruitment challenges is vital for the success of clinical trials, and bioaccess's strategies in this area further amplify its value proposition within the development landscape.
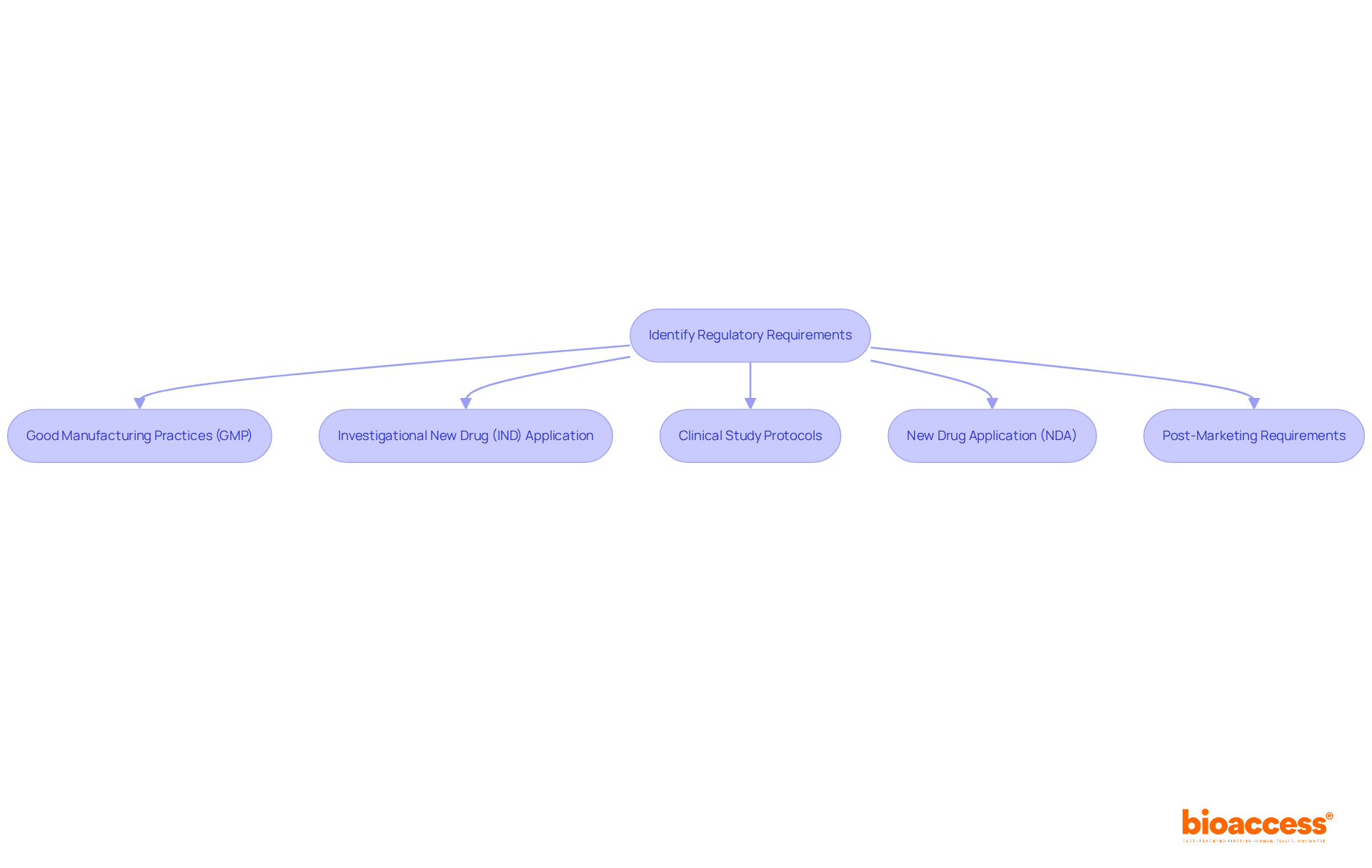
Regulatory requirements for the process of drug approval differ by region and drug type, yet they generally encompass several key components essential for compliance and safety.
Good Manufacturing Practices (GMP) are crucial for all pharmaceutical manufacturers, ensuring that medications are consistently produced and regulated according to strict quality standards. In 2025, it was observed that most companies inspected by the FDA were fully compliant with these regulations, underscoring the importance of maintaining robust quality management systems.
The Investigational New Drug (IND) Application is a critical step before initiating clinical studies. Sponsors are required to submit an IND application to the FDA, detailing the drug's composition, manufacturing processes, and proposed clinical study protocols, thereby laying the groundwork for regulatory approval.
Each clinical study must adhere to meticulously crafted Clinical Study Protocols that outline the study's objectives, design, methodology, statistical considerations, and ethical compliance. This structured approach is vital for ensuring the integrity and reliability of trial outcomes. In Colombia, this includes obtaining study approval from the site's institutional review board (IRB) or ethics committee (EC), as well as from the regulatory agency INVIMA and the Ministry of Health (MoH).
The New Drug Application (NDA) serves as a comprehensive document encompassing all data from preclinical and clinical studies, suggested labeling, and detailed information on the substance's manufacturing process. This submission is vital for the FDA's assessment of the medication's safety and effectiveness.
Finally, Post-Marketing Requirements may necessitate that companies conduct further studies or provide regular safety reports to monitor the medication's long-term effects. This ongoing oversight is crucial for ensuring continued compliance and patient safety.
Understanding these regulatory requirements early in the development phase is essential for simplifying the process of drug approval and ensuring compliance with industry standards. Furthermore, leveraging services such as feasibility studies, site selection, and project management can significantly enhance the efficiency of navigating the regulatory landscape.
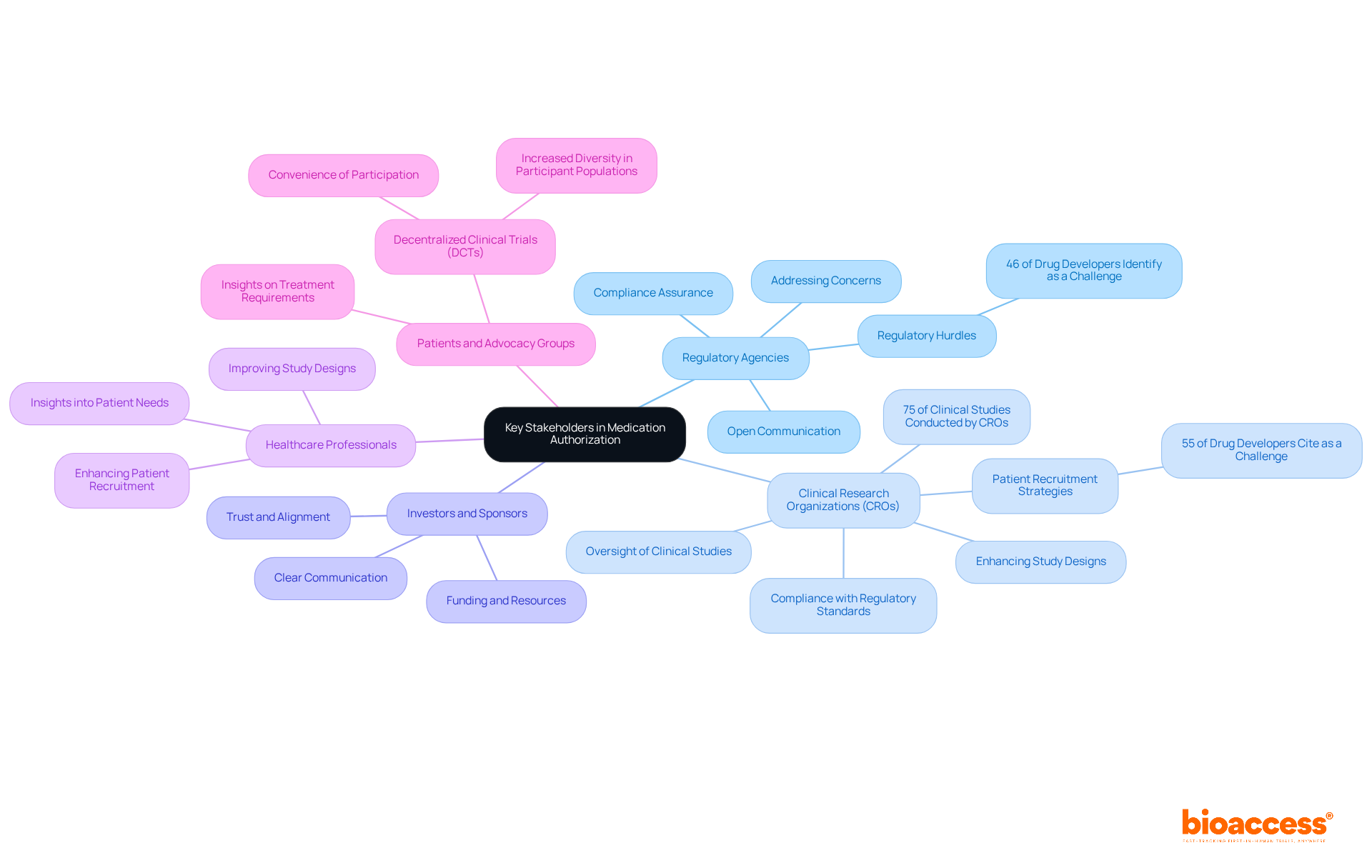
Efficient cooperation with important stakeholders is essential for successful medication authorization. The primary stakeholders include:
Establishing open lines of communication with regulatory agencies is essential to ensure compliance and promptly address any concerns that may arise during the process of drug approval. This proactive approach can significantly reduce regulatory hurdles, which 46% of drug developers identify as a top challenge.
Clinical Research Organizations (CROs): Collaborating with CROs provides specialized knowledge in overseeing clinical studies, ensuring compliance with regulatory standards, and enhancing study designs. With almost 75% of clinical studies carried out by CROs, their role in improving study efficiency and adherence is essential. CROs also implement targeted outreach strategies to address patient recruitment challenges, which 55% of drug developers cite as a significant issue.
Investors and Sponsors: Keeping investors informed about progress and challenges is vital, as their support is crucial for funding and resources. Clear communication fosters trust and ensures alignment on project goals.
Healthcare Professionals: Engaging with healthcare providers can yield valuable insights into patient needs and treatment outcomes, informing study designs and improving patient recruitment strategies. This engagement can improve the overall quality of clinical studies.
Patients and Advocacy Groups: Engaging patients in the development phase offers vital insights on treatment requirements and can greatly improve recruitment efforts. Decentralized clinical trials (DCTs) facilitate better patient engagement by allowing participation from their own locations, thus increasing diversity in participant populations.
To foster collaboration, establish regular meetings, utilize project management tools, and create a shared vision among all stakeholders. This organized method will simplify the process of drug approval and improve the chances of success, ultimately resulting in more efficient medication development.
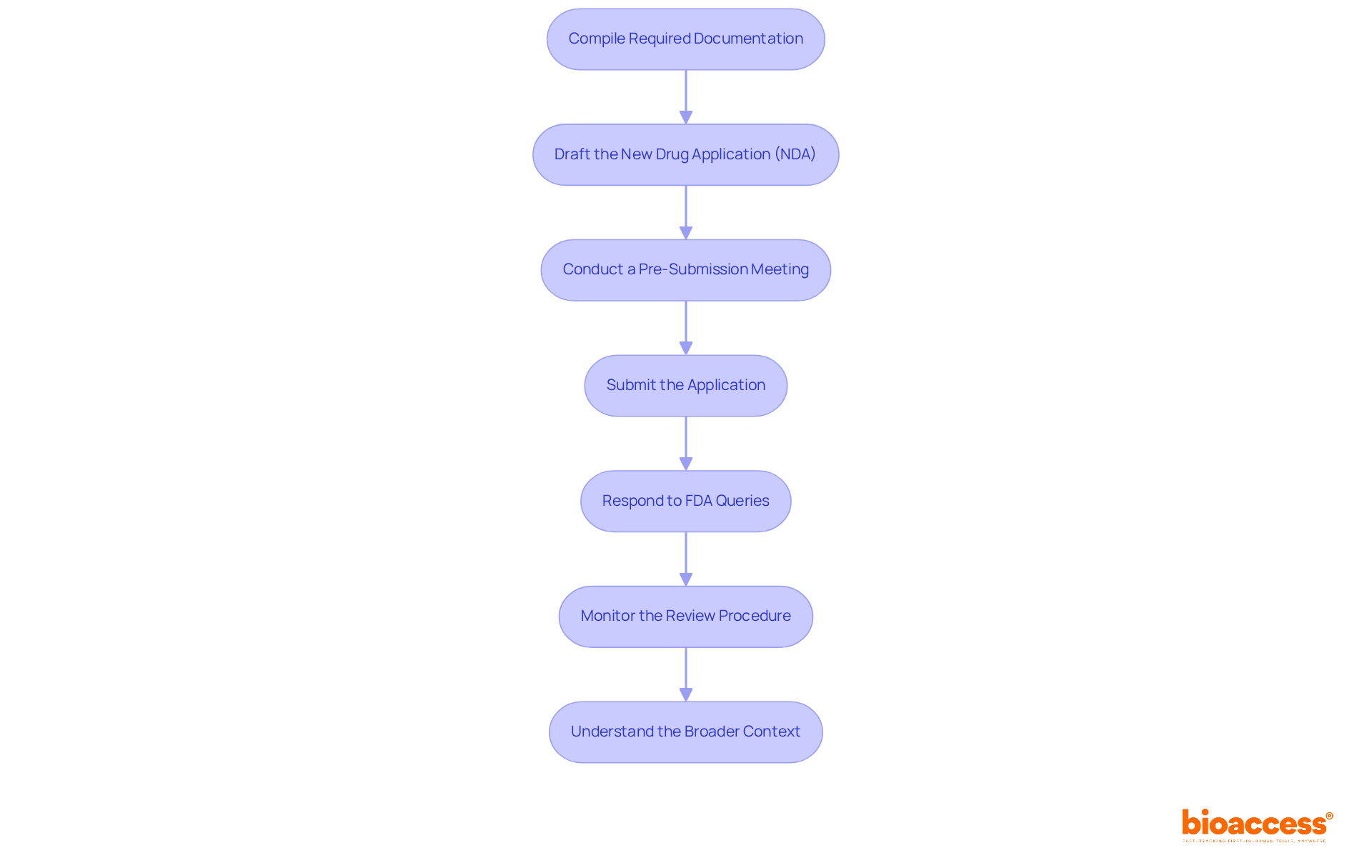
The final step in the process of drug approval involves meticulous preparation and submission of your application. Follow these essential steps, leveraging bioaccess's comprehensive clinical trial management services to enhance your submission:
Compile Required Documentation: Gather all necessary documents, including clinical trial data, manufacturing processes, labeling, and safety information. Ensure that all data is accurate and current, as a well-organized NDA significantly increases the likelihood of first-cycle approval, reducing the risk of delays.
Draft the New Drug Application (NDA): The NDA must encompass comprehensive information about the drug, detailing its pharmacology, toxicology, and clinical efficacy. Pay close attention to the formatting and organization of the application, as clarity is paramount. The NDA should adhere to the Common Technical Document (CTD) format, which organizes submissions into five key modules.
Conduct a Pre-Submission Meeting: Schedule a pre-submission meeting with the FDA to discuss your application and receive valuable feedback. This proactive step can help identify potential issues before formal submission, enhancing the overall quality of your application.
Submit the Application: Once the NDA is finalized, submit it electronically through the FDA's Electronic Submissions Gateway (ESG). Ensure you obtain confirmation of receipt, as this signifies the official beginning of the review.
Respond to FDA Queries: After submission, be prepared to promptly address any questions or requests for additional information from the FDA. This may involve providing further data or clarifications, which is crucial since the FDA typically has 60 days to determine if the application is complete and ready for formal review.
Monitor the Review Procedure: Stay engaged with the FDA throughout the review. Proactively addressing any concerns that arise can help expedite the approval timeline. The typical FDA evaluation period lasts around 10 months during standard review, but priority review can reduce this to roughly 6 months for medications that provide substantial advancements over current treatments.
Understand the Broader Context: Recognize that the NDA submission is the culmination of years of pharmaceutical development and clinical trials, which typically take 10-15 years from discovery to market. Furthermore, post-marketing monitoring and continuous regulatory adherence are essential elements of the medication endorsement system that must be upheld to guarantee long-term safety and effectiveness.
By adhering to these steps and maintaining open communication with regulatory agencies, you can significantly enhance your chances of achieving a successful process of drug approval. Furthermore, leveraging bioaccess's comprehensive clinical trial management services, such as feasibility studies, site selection, compliance reviews, trial setup, import permits, project management, and reporting, can streamline the process and ensure adherence to regulatory requirements.
Mastering the drug approval process is crucial for pharmaceutical developers aiming to bring innovative therapies to market. This comprehensive guide outlines the essential steps and knowledge required to navigate the complex landscape of medication authorization, emphasizing the significance of understanding regulatory bodies, development phases, and collaboration with key stakeholders.
The article delves into the various phases of drug development, from initial discovery to post-market surveillance, highlighting the critical nature of each stage in ensuring safety and efficacy. Key insights into regulatory requirements and the importance of strategic partnerships illustrate how adherence to guidelines and effective communication can streamline the approval process. Moreover, the evolving nature of regulatory expectations, particularly the FDA's openness to real-world evidence, signals a shift that may enhance the speed and efficiency of future drug approvals.
In conclusion, the journey of drug approval is not merely a regulatory hurdle but a vital pathway to delivering safe and effective treatments to patients in need. By fostering collaboration among stakeholders, leveraging innovative technologies, and staying informed about regulatory changes, developers can significantly improve their chances of success. Embracing these strategies not only accelerates the introduction of new medications but also contributes to the broader goal of advancing public health.
What is the primary authority governing medication approval in the United States?
The primary authority governing medication approval in the United States is the U.S. Food and Drug Administration (FDA).
What are the different categories of medication authorizations?
The two main categories of medication authorizations are New Drug Applications (NDA) for new molecular entities and Abbreviated New Drug Applications (ANDA) for generic drugs.
What is the expected trend for new medication authorizations in 2025?
In 2025, it is expected that approximately 40% of new medication authorizations will proceed through the 505(b)(2) pathway, which allows for a more efficient drug approval process by leveraging existing data.
Can you provide an example of a successful medication authorization by the FDA?
An example of a successful medication authorization by the FDA is Spravato (esketamine), which was endorsed via the 505(b)(2) pathway to address new therapeutic needs.
What phases are involved in the drug development process?
The drug development process involves several critical phases: discovery and development, preclinical research, clinical trials (which are further divided into Phase 1, Phase 2, and Phase 3), FDA review, and post-market surveillance.
What is the focus of Phase 1 clinical trials?
Phase 1 clinical trials primarily focus on evaluating the safety of the drug and its effects on a small group of healthy volunteers.
What is the average time it takes to introduce a medication to the market?
The average time to introduce a medication to the market exceeds 10 years.
What percentage of authorized medications may be removed from the market post-approval?
Approximately 4%-6% of authorized medications may be removed from the market due to safety concerns that were not detected during clinical evaluations.
How are recent trends influencing clinical trial methods?
Recent trends indicate that sponsors are increasingly utilizing big data and innovative technologies to enhance clinical trial methods, aiming to shorten timelines and boost efficiency.
What advantages does bioaccess offer in the regulatory approval process?
Bioaccess offers an accelerated regulatory approval process that enables approval in just 6-8 weeks, compared to the typical 6-12 months in the US/EU, facilitating faster enrollment of treatment-naive cohorts and benefiting local economies.