


Understanding the complexities of the National Agency for Medicines and Medical Devices (NAMMD) framework is crucial for anyone involved in the pharmaceutical industry. As Romania positions itself as a key player in clinical research, this guide highlights the essential timelines for drug approval under NAMMD. Mastering the regulatory landscape not only accelerates the authorization process but also enhances strategic planning for stakeholders.
With recent updates poised to reshape these timelines by December 2025, stakeholders must consider how to adapt their strategies effectively. How can they navigate this evolving framework to ensure timely medication approvals? This question underscores the importance of staying informed and proactive in an ever-changing environment.
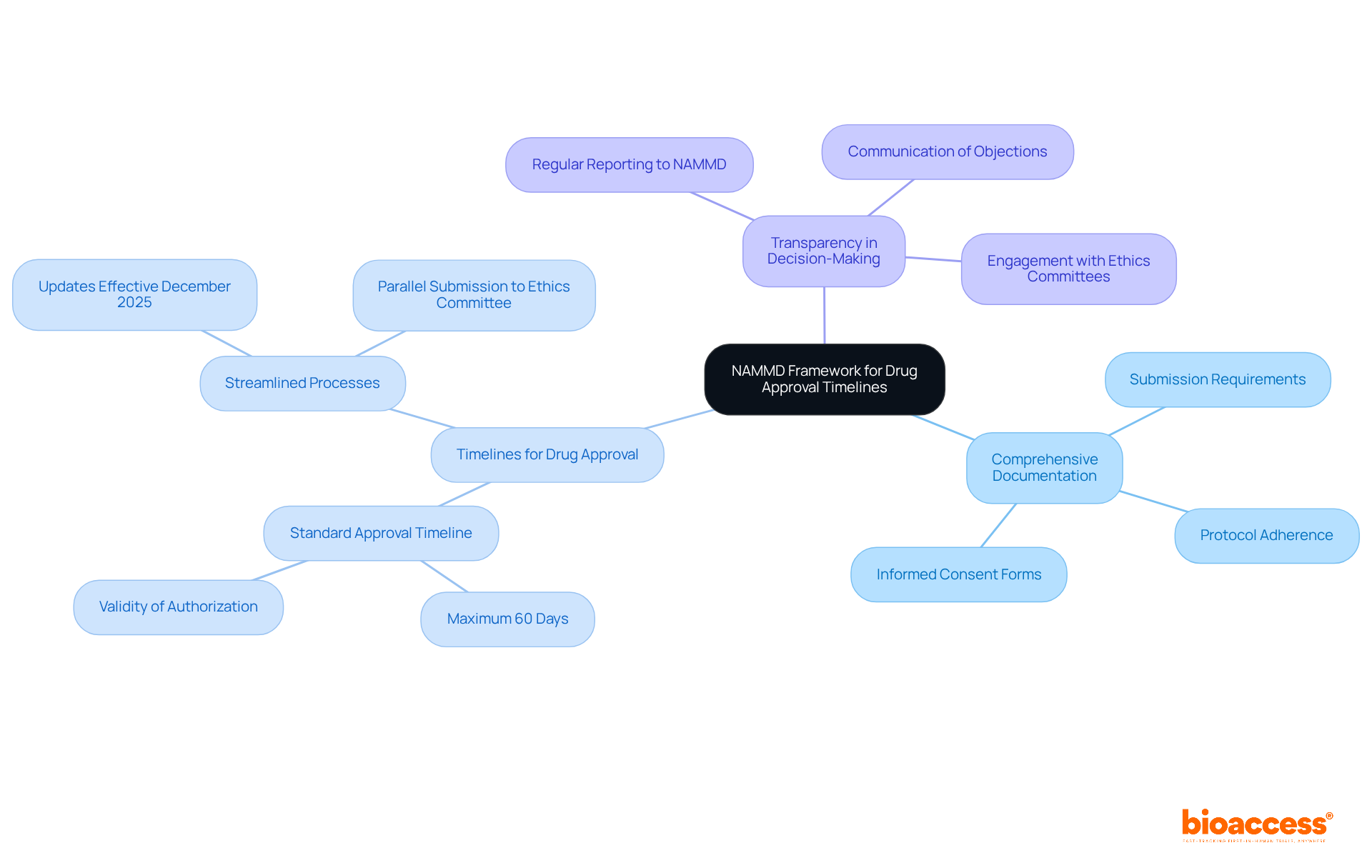
The National Agency for Medicines and Medical Devices (NAMMD) serves as Romania's primary regulatory authority for medication certifications, playing a pivotal role in ensuring compliance with both national and EU regulations. Understanding this framework is crucial for effectively navigating the drug authorization system. The agency upholds Good Clinical Practice (GCP) and ethical standards, which are vital for preserving the integrity of clinical trials.
Key components of the NAMMD framework include:
Recent updates to the regulatory framework, set to take effect in December 2025, have streamlined timelines for drug approval under NAMMD, significantly enhancing Romania's attractiveness for clinical research. Grasping these elements can profoundly influence the speed and success of medication clearances, ultimately fostering the development of innovative treatments in the field.
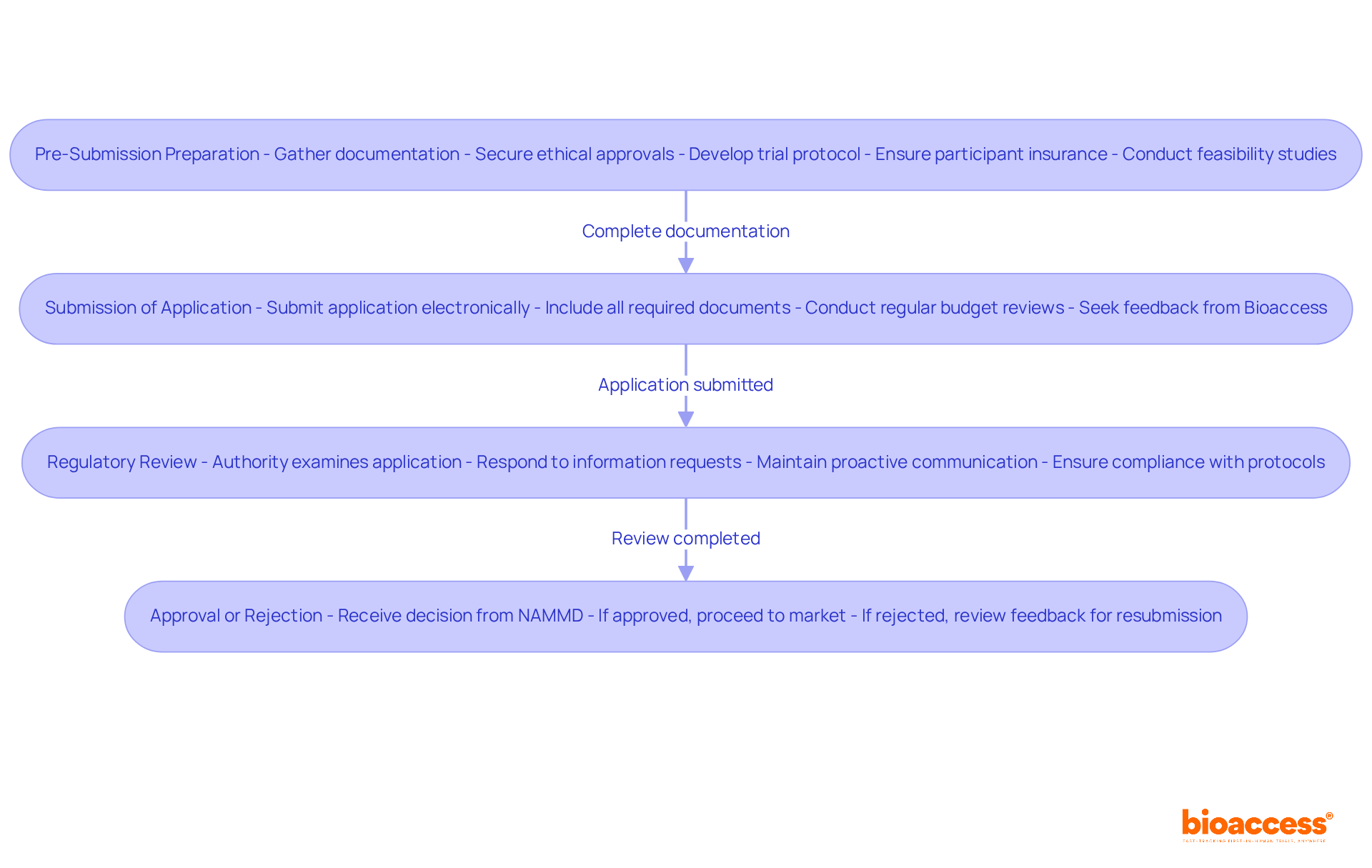
The drug approval process is a multifaceted journey that requires meticulous attention at every stage, particularly when considering the timelines for drug approval under NAMMD.
Pre-Submission Preparation is the cornerstone of success. This phase involves gathering essential documentation, including clinical trial data, ethical approvals, and a comprehensive trial protocol. A well-crafted protocol not only outlines research objectives and methodologies but also establishes participant selection criteria, ensuring compliance with Romanian legislation and international standards. Moreover, securing insurance protection for research participants is vital, as it safeguards them against potential injuries or adverse effects during the study. Bioaccess provides feasibility studies and site selection services, ensuring that the research site and principal investigator (PI) are optimally suited for the trial.
Once the documentation is complete, the Submission of Application phase begins. The application must be submitted electronically to the relevant authority, with all required documents included to avoid delays. Regular budget reviews during this phase can help identify financial inefficiencies. Bioaccess offers extensive assistance in evaluating and providing feedback on study documents to meet country requirements, enhancing the submission experience.
The Regulatory Review stage involves a thorough examination of the application by the authority, which can take up to 60 days. During this time, additional information or clarifications may be requested. Proactive communication with the organization can mitigate potential delays and enhance the likelihood of a seamless review process. The involvement of qualified investigators is crucial, as their expertise ensures compliance with protocols and participant safety. Bioaccess's project management and monitoring services are instrumental in maintaining compliance throughout this phase, including regular reporting on study status and any adverse events.
Following the review, NAMMD will issue a decision in the Approval or Rejection phase. If approved, the medication can proceed to market; if rejected, detailed feedback will guide resubmission efforts. Understanding these phases and adhering to optimal methods significantly simplifies the authorization process, which ultimately enhances success rates for pharmaceutical applications and aligns with the timelines for drug approval under NAMMD. Continuous adherence and oversight after receiving regulatory consent are essential for preserving trial integrity and ensuring patient safety. Katherine Ruiz, an expert in regulatory affairs for medical devices and in vitro diagnostics in Colombia, leads Bioaccess's efforts in navigating these complex regulatory landscapes.
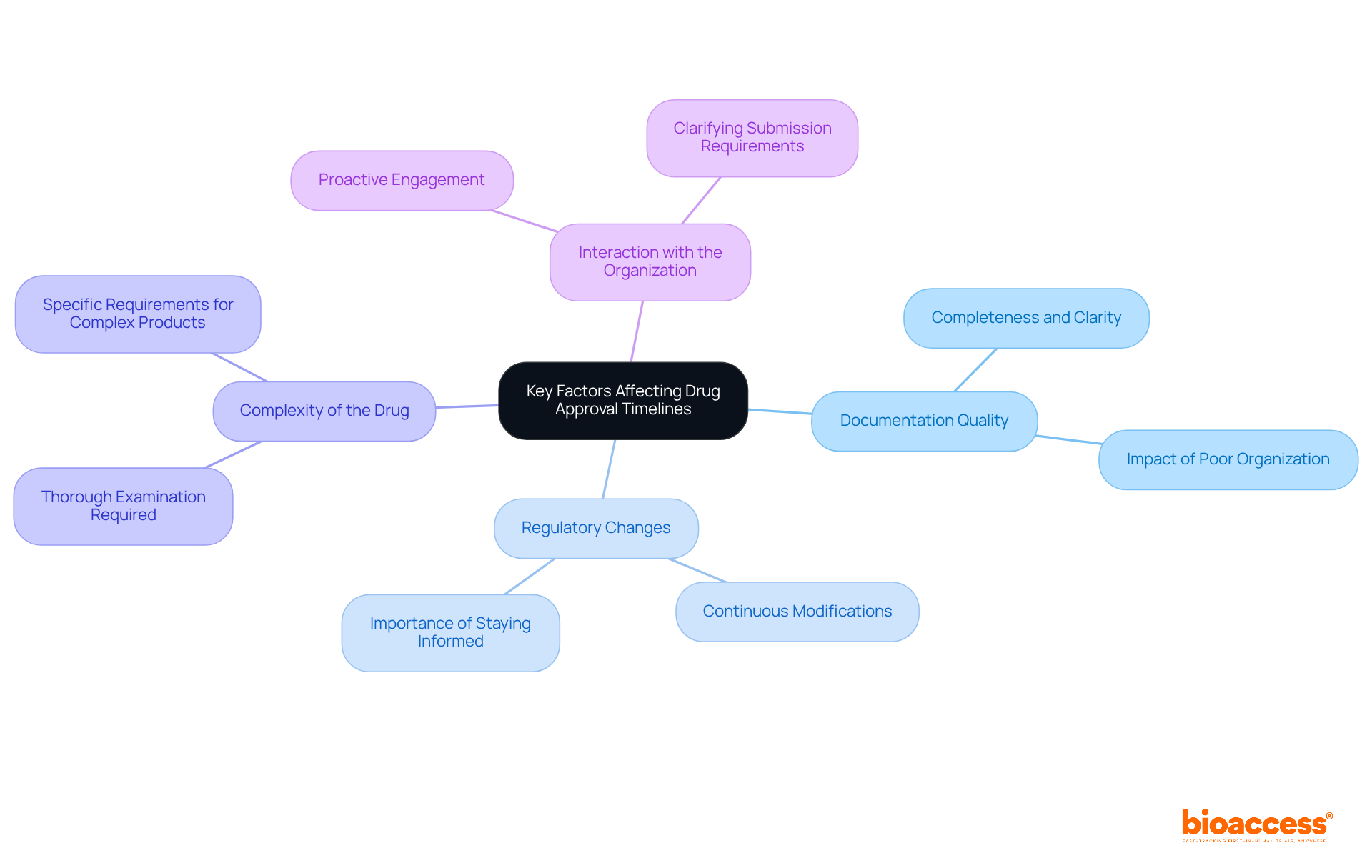
Several key factors significantly influence drug approval timelines under the National Agency for Medicines and Medical Devices (NAMMD):
Documentation Quality: The completeness and clarity of submissions are paramount. Inadequate or poorly organized documentation can lead to substantial delays, as NAMMD requires thorough and precise information to assess applications effectively.
Regulatory Changes: Continuous modifications to regulations and guidelines can influence the endorsement procedure. Staying informed about these changes is essential for timely submissions and compliance.
Complexity of the Drug: Medications with elaborate compositions or novel mechanisms frequently require a more thorough examination, which can prolong endorsement timelines. Understanding the specific requirements for complex products is crucial for efficient processing.
Interaction with the Organization: Engaging proactively with the group can clarify submission requirements and speed up the process of authorization. Regular dialogue helps address potential issues early, ensuring that applications meet all necessary criteria.
By identifying and tackling these elements, stakeholders can improve their approaches for maneuvering through the regulatory landscape to adhere to the timelines for drug approval under NAMMD. This ultimately results in quicker and more successful medication endorsements. How can your organization adapt to these challenges to enhance your submission success?
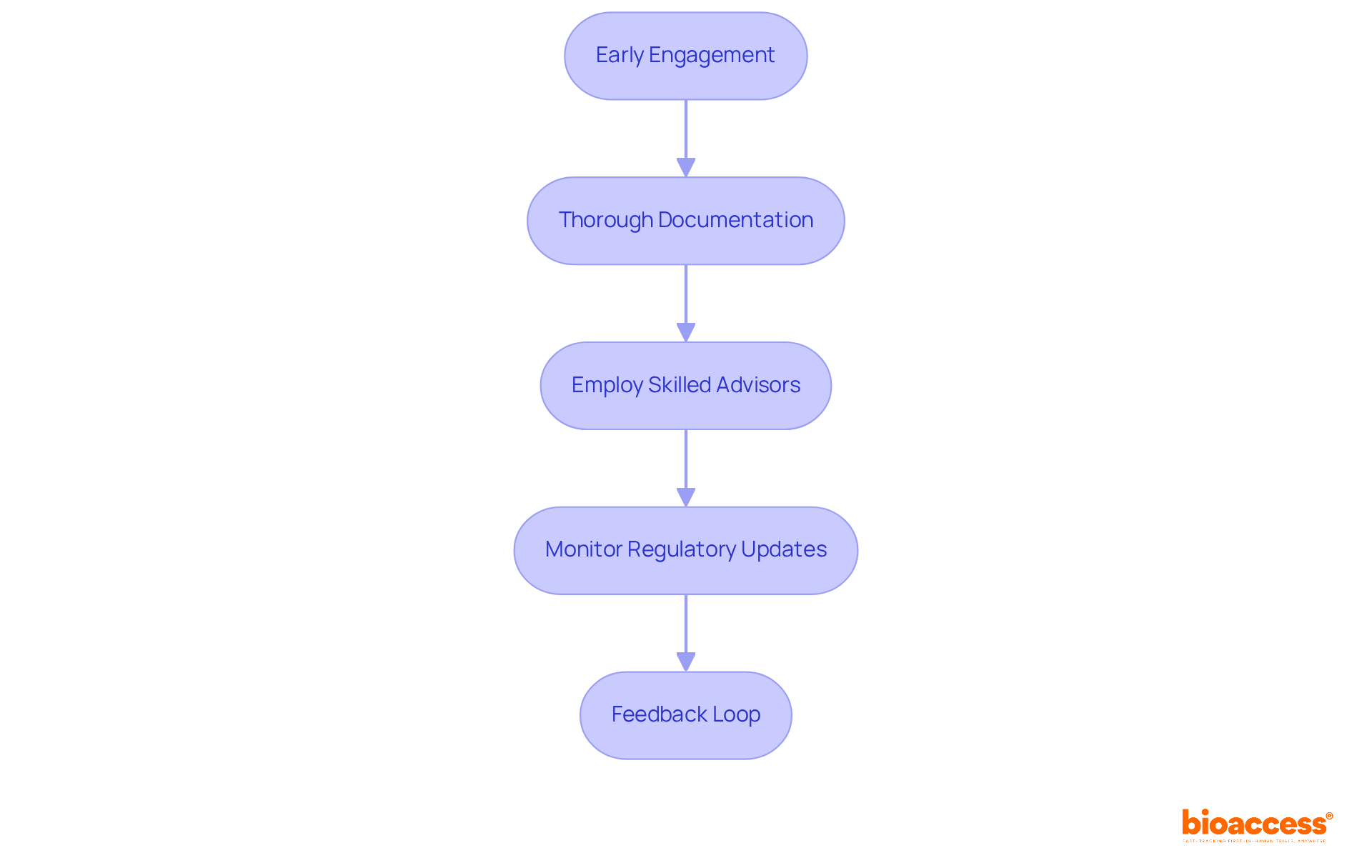
To expedite drug approval under NAMMD, implementing the following strategies is essential:
Early Engagement: Initiating discussions with NAMMD at the outset is crucial. This proactive strategy enables researchers to clarify requirements and expectations, significantly improving the chances of prompt endorsements. Case studies have shown that early involvement can decrease assessment timelines by as much as 30%, promoting a clearer understanding of regulatory expectations.
Thorough Documentation: Comprehensive and well-organized submissions are vital. Research indicates that errors in documentation can lead to delays of up to 60 days. Ensuring that all necessary documents, including the clinical study application and informed consent forms, are meticulously prepared can simplify the authorization process.
Employ Skilled Advisors: Involving regulatory consultants with expertise in medical device processes can provide invaluable guidance. Their knowledge of the regulatory environment assists in navigating challenges and enhancing submission quality, ultimately resulting in quicker authorizations.
Monitor Regulatory Updates: Staying informed about changes in regulations is imperative. The organization regularly revises its guidelines, and being aware of these alterations can prevent compliance issues that might delay authorization.
Feedback Loop: Establishing a feedback system with the organization allows for swift resolution of any issues. Regular communication facilitates smoother interactions and ensures that necessary adjustments to submissions are made promptly.
By adopting these strategies, researchers can significantly reduce timelines for drug approval under NAMMD and improve overall efficiency in the clinical trial process.
Understanding the complexities of the NAMMD framework for drug approval is crucial for stakeholders looking to navigate Romania's regulatory landscape effectively. By mastering the timelines and processes set forth by the National Agency for Medicines and Medical Devices, organizations can significantly boost their chances of securing successful medication endorsements.
This article explores key elements of the drug approval process, emphasizing the necessity of:
It underscores the vital role of meticulous preparation in expediting approvals while addressing critical factors that can impact timelines, such as the drug's complexity and the quality of interactions with NAMMD. Strategies like early engagement and establishing a feedback loop can lead to substantial reductions in approval timelines, ultimately fostering a more efficient clinical trial process.
In summary, the NAMMD framework presents both challenges and opportunities for drug developers. By adopting best practices and adapting to the evolving regulatory environment, organizations can streamline their submission processes and contribute to the advancement of innovative treatments. The future of drug approval in Romania is on the brink of improvement, and active participation in this journey is essential for all stakeholders involved.
What is the NAMMD and what role does it play in Romania?
The National Agency for Medicines and Medical Devices (NAMMD) is Romania's primary regulatory authority responsible for medication certifications, ensuring compliance with national and EU regulations.
Why is understanding the NAMMD framework important?
Understanding the NAMMD framework is crucial for effectively navigating the drug authorization system and ensuring compliance with Good Clinical Practice (GCP) and ethical standards in clinical trials.
What are the key components of the NAMMD framework?
Key components include the submission of comprehensive documentation, adherence to timelines for drug approval, and a commitment to transparency in decision-making.
What recent updates have been made to the NAMMD regulatory framework?
Recent updates, set to take effect in December 2025, have streamlined timelines for drug approval, enhancing Romania's attractiveness for clinical research.
How do the NAMMD framework elements influence drug approvals?
Grasping the elements of the NAMMD framework can significantly influence the speed and success of medication clearances, promoting the development of innovative treatments.