


The landscape of medical research in Bosnia and Herzegovina is experiencing a pivotal transformation, fueled by the introduction of transparency laws that protect participant rights and bolster accountability. As the nation faces the lowest rate of medical studies in the Balkans, grasping these regulations is essential for researchers aiming to navigate the intricate ethical landscape.
With lengthy approval processes and stringent compliance requirements in place, how can researchers ensure they meet these standards while simultaneously fostering trust and credibility in their findings?
The transparency laws for trial data in Bosnia and Herzegovina play a vital role in fostering accountability and integrity within medical research. These regulations mandate that all medical studies operate with a high degree of openness, ensuring that information is readily available and that participants' rights are safeguarded. A fundamental aspect of this framework is the Law on Protection of Personal Data, which aligns with GDPR standards. This regulation stipulates that all personal information collected during experiments must be managed with strict confidentiality and transparency, thereby reinforcing the ethical conduct of research.
Notably, Bosnia and Herzegovina has the lowest number of medical studies in the Balkans, highlighting the urgent need for improved ethical oversight and transparency initiatives. The lengthy approval periods from local ethics boards, which can range from 1 to 15 months, further complicate the research landscape. Understanding the transparency laws for trial data in Bosnia is crucial for compliance and for building trust among stakeholders, including participants, sponsors, and regulatory bodies.
As Bosnia navigates its medical research environment, the implementation of transparency laws for trial data in Bosnia will be essential for enhancing the credibility of its research studies and improving patient care outcomes. The call for action is clear: strengthening these frameworks not only supports ethical research practices but also paves the way for a more trustworthy and effective medical research landscape.
In Bosnia, the regulatory framework for research data transparency is governed by several critical documents, notably the Ordinance on Clinical Studies on Medicinal Products and Medical Devices and the Law on Personal Data Protection. These regulations mandate that all medical studies be registered in a public database, with outcomes disclosed openly to promote accountability. Researchers must anonymize data to safeguard participant identities while providing sufficient detail for independent verification of findings. Adherence to ethical standards set by local ethics committees is crucial, ensuring that studies are conducted with integrity and respect for participants' rights.
As of 2025, ongoing updates to the transparency laws for trial data in Bosnia reflect a commitment to enhancing the ethical standards and transparency of medical research, positioning the country as a potential hub for innovative studies. Notably, Bosnia has a research study per capita rate of 0.67e-4, indicating significant potential for expansion in this sector. Expert insights suggest that addressing bureaucratic challenges in the registration process could further facilitate this potential.
bioaccess offers extensive clinical study management services, including:
By leveraging these services, researchers can effectively navigate the complex regulatory landscape, ensuring compliance with local laws and ethical standards. This ultimately transforms lives through advanced medtech, highlighting the importance of collaboration and proactive engagement in the clinical research arena.
To effectively manage trial data transparency, researchers must adopt several best practices:
Data Registration: Ensure all studies are registered in a public database prior to initiation. This step is crucial for accountability and allows for public scrutiny of the research process.
Regular Reporting: Establish a consistent schedule for reporting study results, including interim findings. This method not only upholds clarity but also ensures that stakeholders are aware of the progress and results of the experiment.
Anonymization Protocols: Implement robust anonymization techniques to protect participant identities while facilitating data sharing. This balance is essential for ethical compliance and fosters trust among participants and the public.
Stakeholder Communication: Maintain open lines of communication with all stakeholders, including participants, sponsors, and regulatory bodies. Engaging stakeholders early and often enhances the relevance and impact of the research, ensuring that their insights shape the trial's direction.
Training and Compliance Checks: Regularly educate staff on compliance requirements and conduct audits to ensure adherence to openness laws. This proactive approach not only reduces risks of non-compliance but also strengthens a culture of openness within the research team.
By applying these best practices, researchers can effectively manage the complexities of transparency laws for trial data in Bosnia, thereby fostering trust and enhancing the credibility of their findings.

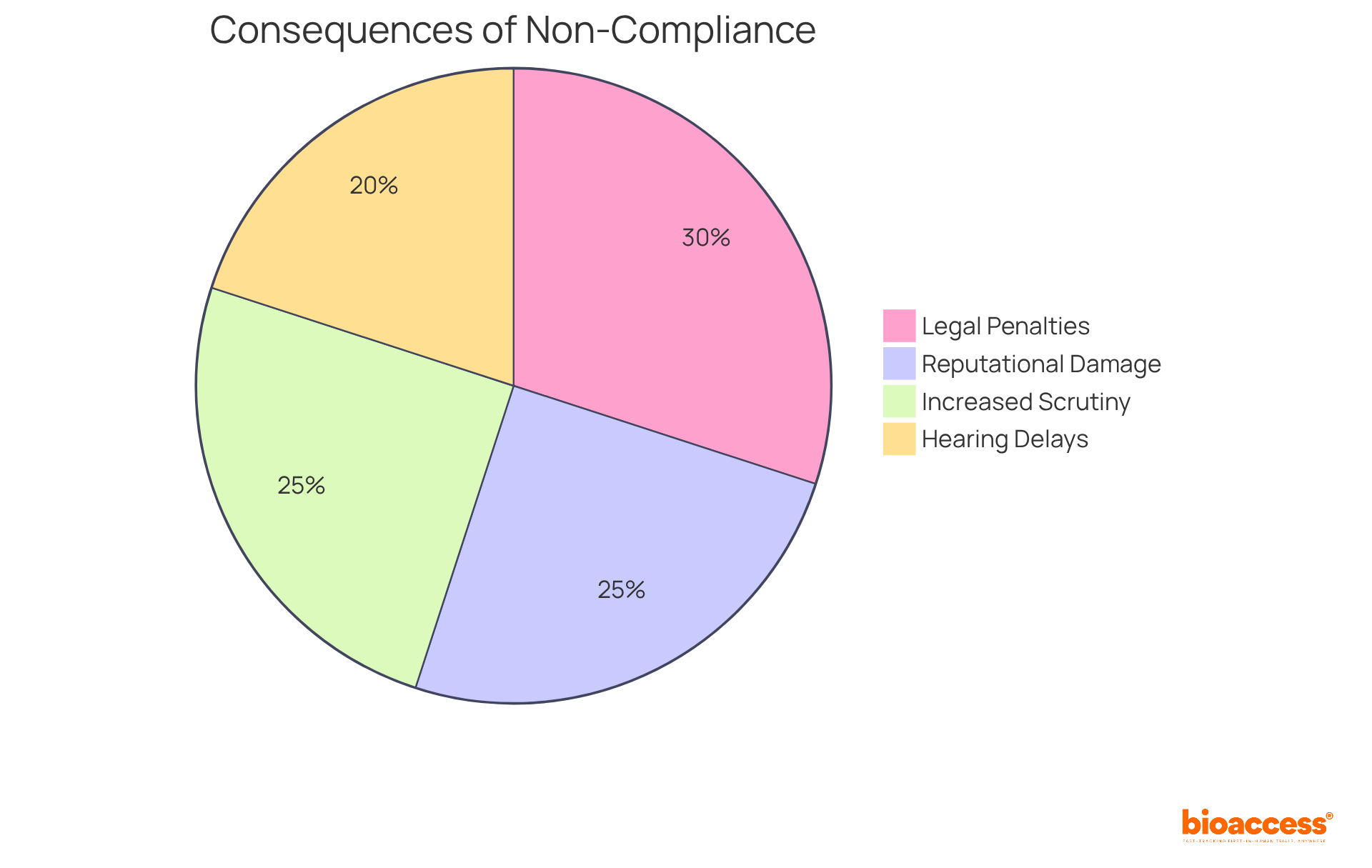
Non-compliance with transparency regulations in Bosnia can lead to significant repercussions:
Legal Penalties: Organizations may incur fines of up to BAM 100,000 (approximately EUR 50,000) for breaches of data protection regulations, reflecting the stringent regulatory environment.
Reputational Damage: A failure to comply with these regulations can erode trust among participants and stakeholders, severely impacting the organization's credibility and future partnerships.
Hearing Delays: Non-compliance frequently leads to postponements in approval processes and participant recruitment, disturbing project schedules and increasing expenses.
Increased Scrutiny: Organizations may attract heightened scrutiny from regulatory authorities, leading to more frequent audits and oversight, which can strain resources and operational efficiency.
Recognizing these consequences underscores the necessity of strict adherence to transparency laws to facilitate the successful execution of clinical trials. By leveraging bioaccess's services, such as compliance reviews and project management, organizations can effectively navigate these challenges, ensuring adherence to regulations and minimizing risks associated with non-compliance.
Implementing transparency laws for trial data in Bosnia is crucial for cultivating a trustworthy and ethical medical research environment. By mandating open access to information and protecting participants' rights, these regulations not only enhance accountability but also aim to elevate the overall quality of research conducted in the country. As Bosnia strives to improve its position in the medical research landscape, understanding and adhering to these laws becomes a vital factor for success.
Key insights from this guide emphasize the necessity of compliance with regulatory requirements, including:
The repercussions of non-compliance can be severe, encompassing legal penalties and reputational damage, which further highlight the need for researchers to adopt best practices. By fostering clear communication with stakeholders and committing to ongoing training and compliance checks, researchers can effectively navigate the complexities of transparency laws.
Ultimately, a commitment to transparency in trial data not only bolsters the credibility of medical research in Bosnia but also acts as a catalyst for innovation and enhanced patient care. As the landscape evolves, stakeholders are encouraged to actively engage with these regulations and embrace the spirit of transparency, ensuring that Bosnia emerges as a recognized hub for ethical and impactful medical studies.
What is the purpose of transparency laws in Bosnia and Herzegovina regarding medical research?
The transparency laws aim to foster accountability and integrity within medical research by ensuring that all studies operate with a high degree of openness and that participants' rights are safeguarded.
What is a key regulation that supports transparency in medical research in Bosnia?
The Law on Protection of Personal Data is a key regulation that aligns with GDPR standards, mandating that all personal information collected during experiments must be managed with strict confidentiality and transparency.
How does Bosnia and Herzegovina's number of medical studies compare to other countries in the Balkans?
Bosnia and Herzegovina has the lowest number of medical studies in the Balkans, indicating a need for improved ethical oversight and transparency initiatives.
What challenges do researchers face in Bosnia regarding the approval of medical studies?
Researchers face lengthy approval periods from local ethics boards, which can range from 1 to 15 months, complicating the research landscape.
Why is understanding transparency laws for trial data important in Bosnia?
Understanding these laws is crucial for compliance and for building trust among stakeholders, including participants, sponsors, and regulatory bodies.
What impact do transparency laws have on medical research credibility in Bosnia?
The implementation of transparency laws is essential for enhancing the credibility of research studies and improving patient care outcomes in Bosnia.
What is the call to action regarding transparency laws in Bosnia's medical research landscape?
The call to action emphasizes the need to strengthen transparency frameworks to support ethical research practices and create a more trustworthy and effective medical research environment.