


Navigating the complex landscape of clinical research in Serbia demands a solid understanding of trial insurance and indemnity laws. These are not just regulatory checkboxes; they are vital safeguards for participant welfare. As Serbia positions itself as a key hub for medical studies in Europe, it’s crucial for sponsors to recognize the significant role that comprehensive insurance coverage plays. This coverage not only mitigates legal risks but also ensures compliance with national regulations. Yet, given the complexities of these laws and the potential for substantial financial implications, how can sponsors effectively balance the need for robust protection with the realities of their research budgets?
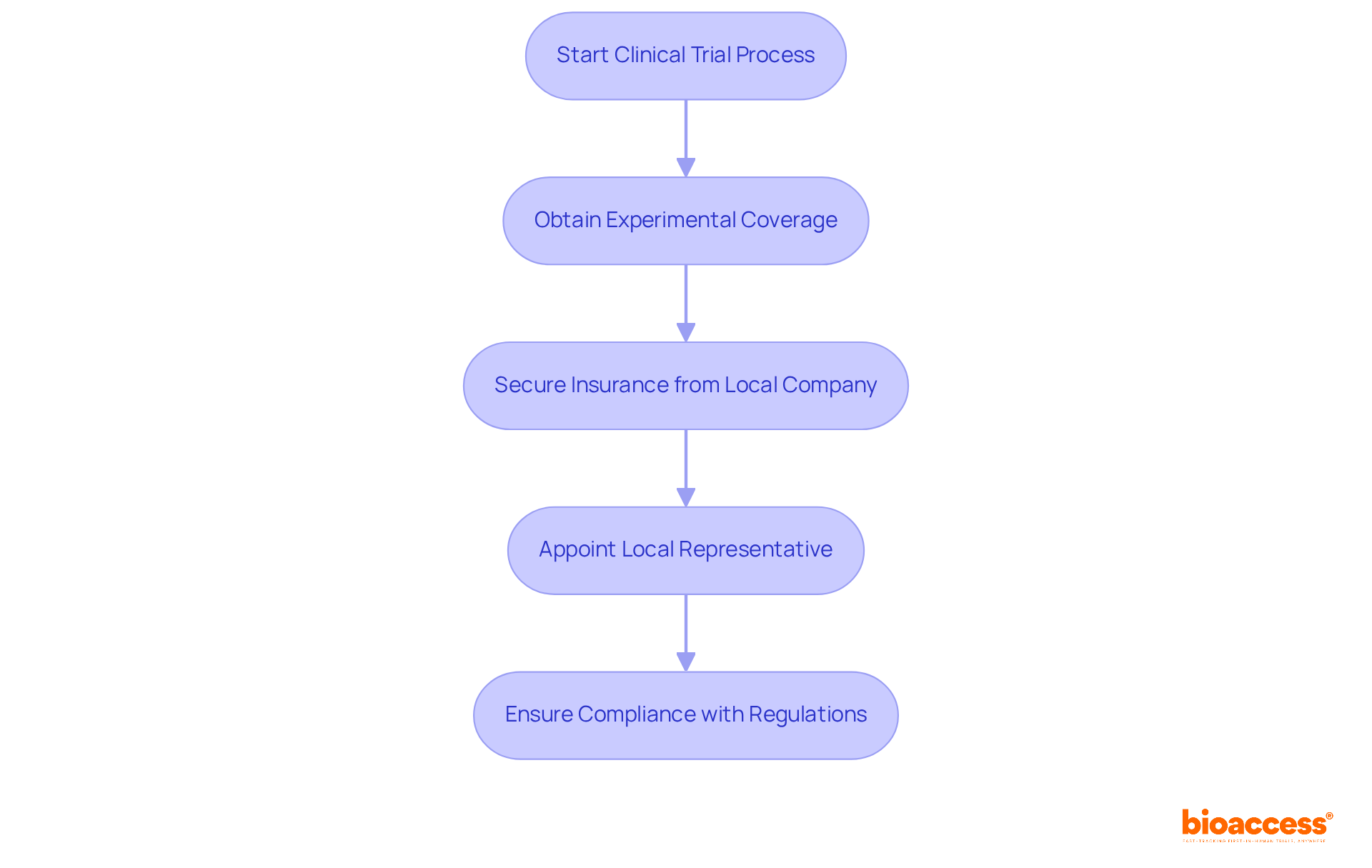
In Serbia, obtaining experimental coverage is not just a formality; it’s a critical requirement for clinical research, as mandated by the Serbian Law on Medicines and Medical Devices. Sponsors must secure coverage for participants to protect against potential health risks associated with the trial. This insurance, issued by a local company, ensures compliance with national regulations. Typically, coverage amounts range from EUR 500,000 to EUR 1,000,000 per occurrence, which is deemed adequate to indemnify participants in case of adverse events. Understanding the trial insurance and indemnity laws in Serbia is vital for sponsors to mitigate legal risks and ensure participant safety.
Furthermore, sponsors are obligated to appoint a local representative who facilitates communication with regulatory bodies and ensures adherence to local laws. This representative is crucial in managing insurance agreements and ensuring that all necessary documentation is prepared before the trial commences. The complexity of these regulations, including trial insurance and indemnity laws in Serbia, underscores the importance of local expertise in navigating the Serbian research landscape, particularly as Serbia positions itself as an emerging hub for medical studies in Europe.
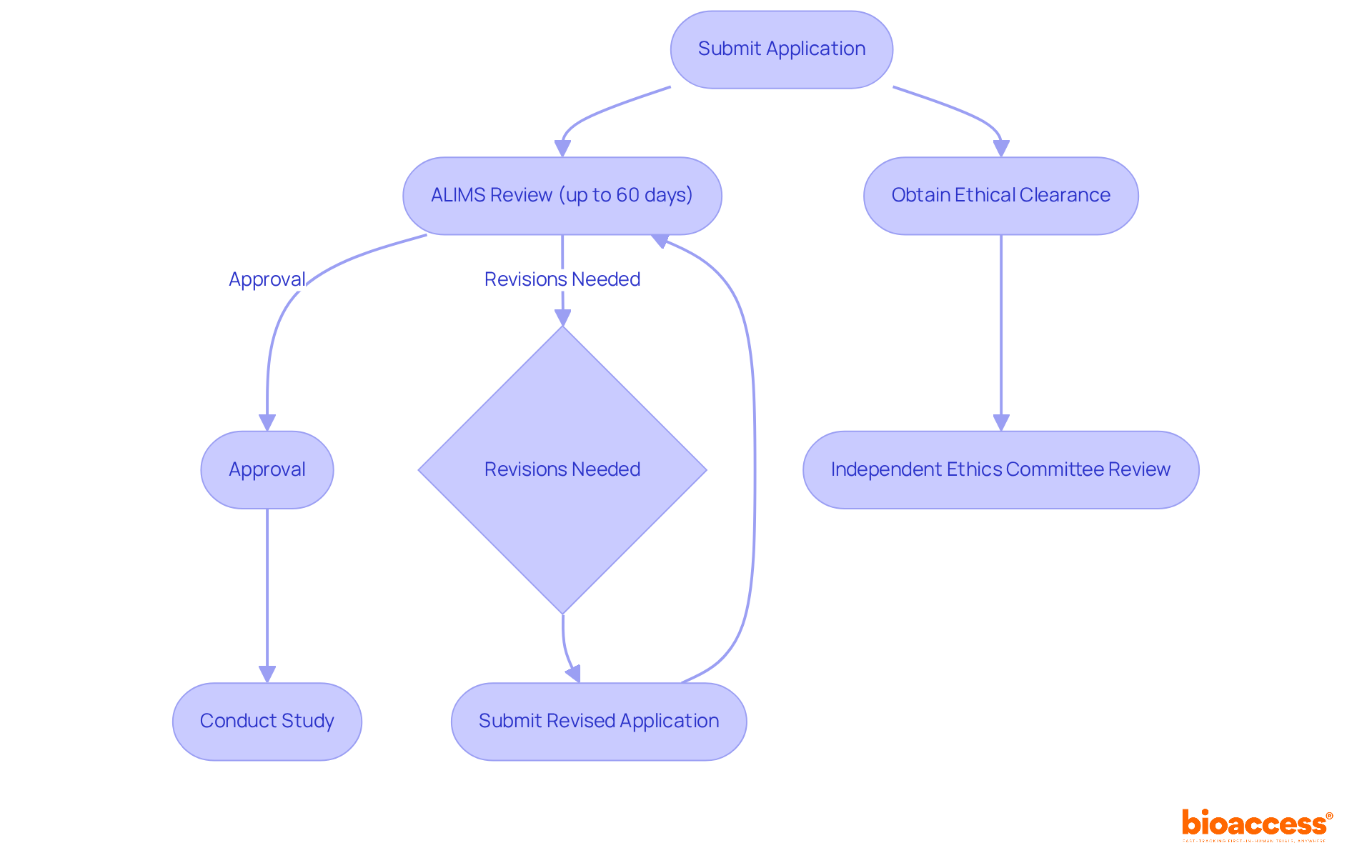
Conducting medical studies in Serbia necessitates adherence to various regulatory systems, primarily overseen by the Medicines and Medical Devices Agency (ALIMS). The approval process begins with sponsors submitting a detailed application that includes the study protocol, informed consent forms, and proof of insurance coverage. Notably, the regulatory process for clinical study applications is efficient, with most approvals completed within a 60-day review period, and some submissions receiving approval in as little as three weeks.
In addition to regulatory approval, obtaining ethical clearance from an independent ethics committee is crucial. This committee assesses the ethical implications of the trial, ensuring that participant rights and welfare are prioritized. Local ethics committees (LECs) in Serbia meet monthly, facilitating timely reviews. Recent amendments to the Clinical Trials Rulebook may impact the approval process and timelines, making it essential for trial sponsors to stay informed about these changes.
Moreover, sponsors based outside Serbia must designate a Local Representative to effectively navigate the regulatory landscape. bioaccess® offers expedited research study services, including site activation in under eight weeks, enabling sponsors to progress swiftly to the next phase of their investigations. Successful examples of medical study applications submitted to ALIMS highlight the efficiency of this regulatory framework. For instance, a recent study conducted by bioaccess® successfully screened 18 patients within four weeks, showcasing the potential for rapid enrollment and effective study execution in Serbia. Understanding these regulatory requirements, along with the support of bioaccess®, is vital for sponsors aiming to navigate the research landscape efficiently.
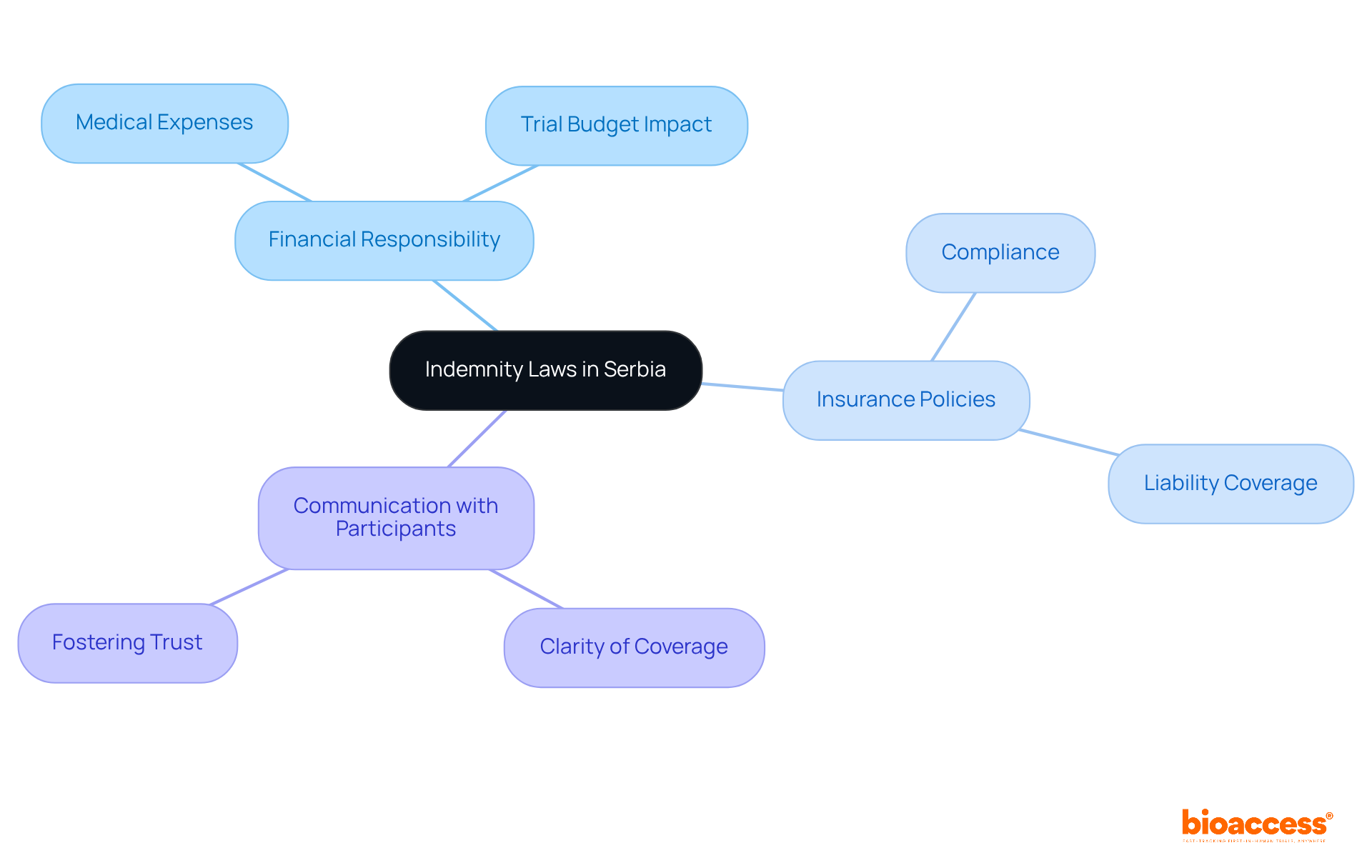
In Serbia, the trial insurance and indemnity laws in Serbia impose a significant obligation on sponsors, mandating complete financial responsibility for any damages incurred during clinical studies. This responsibility extends to covering medical expenses for participants who may suffer adverse effects as a result of the study. It is essential for the policy to clearly define the extent of coverage and the claims process, ensuring clarity for all parties involved.
Understanding the trial insurance and indemnity laws in Serbia is vital for sponsors, as these laws profoundly influence financial planning and risk management strategies for clinical studies. Sponsors must secure robust insurance policies that comply with trial insurance and indemnity laws in Serbia, capable of addressing potential liabilities, which can substantially affect the overall trial budget. Moreover, maintaining transparent communication with participants about their rights and the specifics of coverage is crucial for fostering trust and ensuring ethical conduct throughout the research process.
Understanding trial insurance and indemnity laws in Serbia is crucial for any sponsor engaged in clinical research. These regulations are not just bureaucratic hurdles; they form a framework that ensures participant safety and legal compliance. By securing the right insurance coverage and appointing a local representative, sponsors can adeptly navigate the complexities of conducting clinical trials in Serbia.
This article highlights several key aspects, including:
As Serbia continues to emerge as a hub for clinical research in Europe, understanding and adhering to trial insurance and indemnity laws will be vital for sponsors. By prioritizing compliance and participant welfare, sponsors can not only mitigate legal risks but also foster trust and integrity in their research endeavors. Embracing these practices will ultimately contribute to the advancement of medical studies in Serbia and enhance the overall landscape of clinical trials.
Why is trial insurance important in Serbia?
Trial insurance is critical in Serbia as it is mandated by the Serbian Law on Medicines and Medical Devices to protect clinical research participants against potential health risks associated with the trial.
What coverage amounts are typically required for trial insurance in Serbia?
Coverage amounts for trial insurance in Serbia typically range from EUR 500,000 to EUR 1,000,000 per occurrence, which is considered adequate to indemnify participants in case of adverse events.
Who is responsible for securing trial insurance in Serbia?
Sponsors are responsible for securing trial insurance for participants to ensure compliance with national regulations and to protect against potential health risks.
What role does a local representative play in the clinical trial process in Serbia?
A local representative is obligated to facilitate communication with regulatory bodies, ensure adherence to local laws, manage insurance agreements, and prepare all necessary documentation before the trial commences.
Why is it important to understand trial insurance and indemnity laws in Serbia?
Understanding trial insurance and indemnity laws in Serbia is vital for sponsors to mitigate legal risks and ensure participant safety, especially as Serbia becomes an emerging hub for medical studies in Europe.