


Navigating the complex landscape of local ethics committees in Australia is vital for researchers committed to upholding ethical standards in their work. These committees are instrumental in evaluating research proposals involving human participants, ensuring that moral guidelines are not only met but consistently maintained. This article serves as a comprehensive guide on effectively engaging with these committees, detailing the principles and regulations that govern their operations, the steps for preparing and submitting ethics applications, and strategies for maintaining clear communication throughout the review process.
But what happens when an application encounters unexpected outcomes? How can researchers transform potential setbacks into valuable opportunities for improvement?
Understanding the principles and regulations that govern their operations is essential for effectively working with local ethics committees in Australia. Human Research Ethics Committees (HRECs) are pivotal in evaluating research proposals involving human participants, ensuring compliance with moral standards. Familiarity with the National Statement on Conduct in Human Research is crucial, as it outlines the principles and guidelines researchers must adhere to. The 2025 updates to this statement underscore the necessity for compliance with international standards, including ICH Good Clinical Practice and ISO 14155, thereby reinforcing the commitment to principled research practices.
Moreover, reviewing specific regulations set forth by the National Health and Medical Research Council (NHMRC) and any relevant state-specific guidelines is vital. Understanding these frameworks will enable you to organize your applications and discussions with review boards more effectively, anticipating the types of questions and issues that may arise during the assessment process.
Statistics indicate that compliance rates with moral standards in Australian clinical research are notably high, thanks to working with local ethics committees in Australia, which provide rigorous oversight. Expert opinions suggest that while HRECs have evolved into 'devolved regulators' within the broader regulatory landscape, there remains a pressing need for clear roles and responsibilities to enhance their effectiveness. By leveraging this foundational understanding, you can navigate the complexities of review board interactions and facilitate more seamless approval processes.
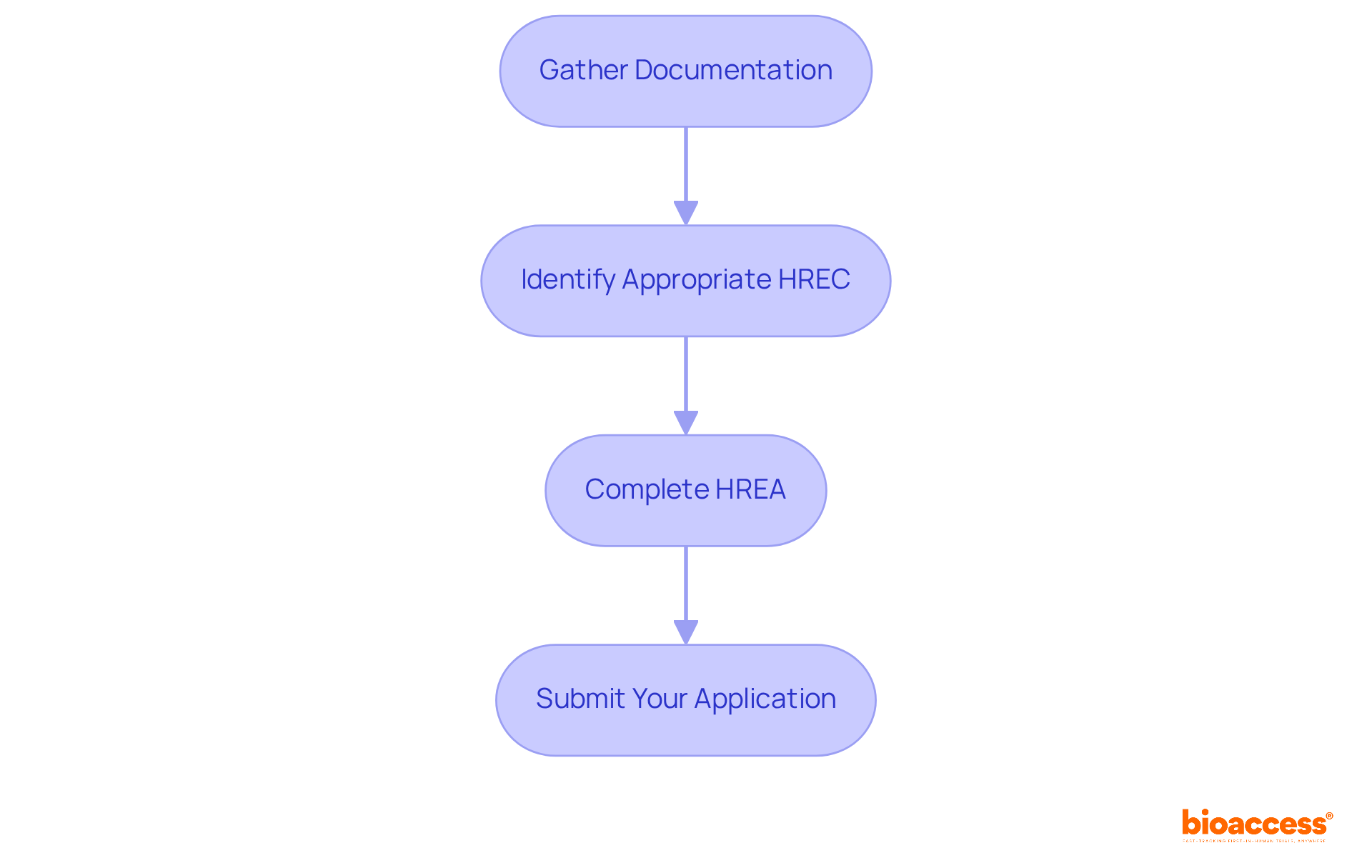
To effectively prepare and submit your ethics request, begin by gathering essential documentation. This includes your research protocol, informed consent forms, and any supplementary materials that reinforce your study's ethical considerations.
By meticulously preparing your application and incorporating expert feedback, you significantly enhance the likelihood of a favorable review while working with local ethics committees in Australia. This paves the way for your research to proceed ethically and efficiently.
In the review process of moral standards, maintaining efficient dialogue with the oversight group is crucial. Here are strategies to enhance your interactions:
By nurturing a cooperative connection with the advisory group, you can navigate the review process more effectively.

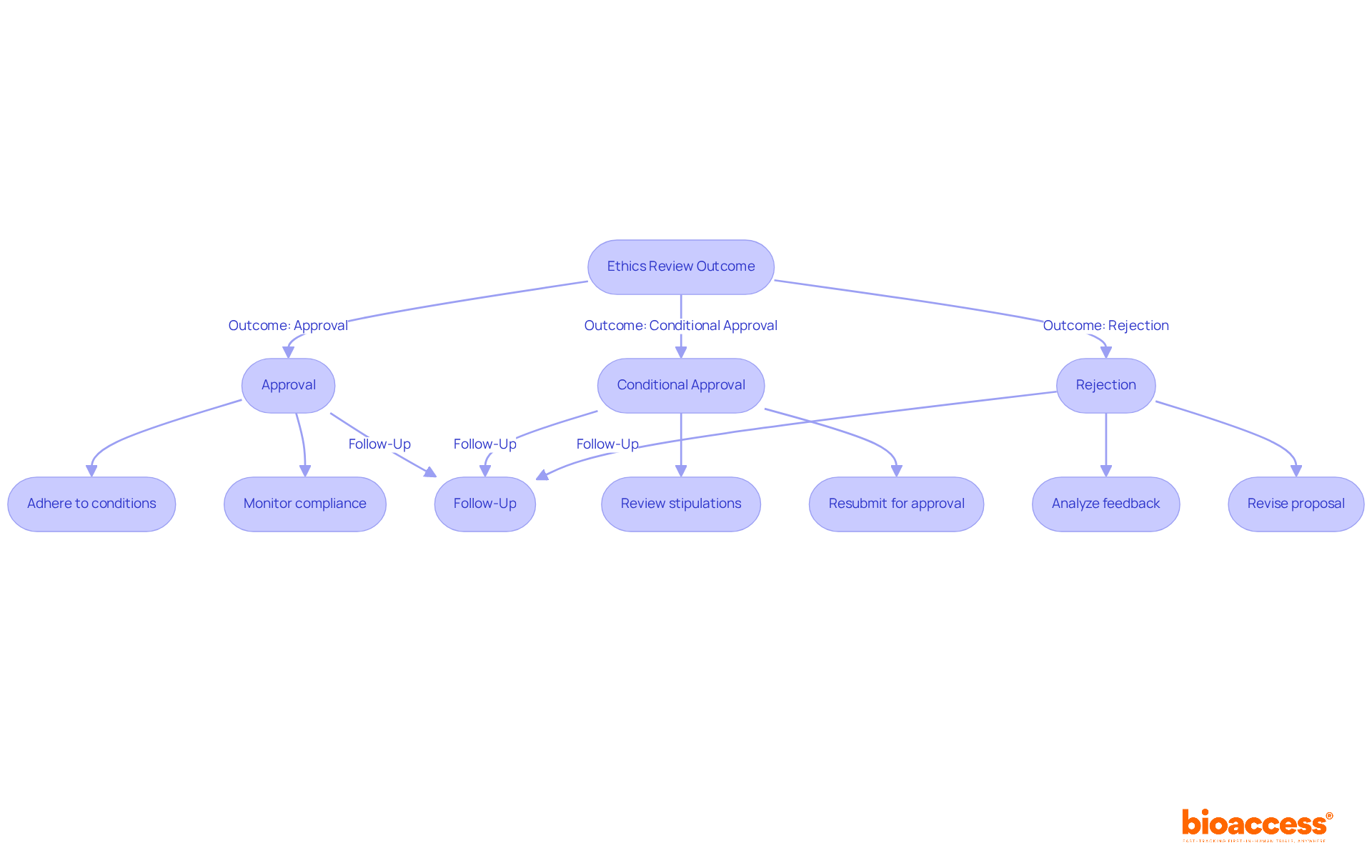
Upon receiving the ethics board's decision regarding your request, which is part of the process of working with local ethics committees in Australia, you may encounter one of three outcomes: approval, conditional approval, or rejection. Understanding how to effectively interpret and respond to each scenario is crucial for your research's success, especially when working with local ethics committees in Australia.
Approval: If your application receives approval, it’s vital to adhere to any conditions set forth by the committee. This may involve modifying your study protocol or implementing additional monitoring measures to ensure compliance with ethical standards. Keep in mind that the median time for ethics approval was 48 days, underscoring the importance of timely adherence to these conditions while working with local ethics committees in Australia.
Conditional Approval: In the event of conditional approval, carefully review the committee's stipulations. Common conditions might include additional data collection, adjustments to the informed consent process, or enhanced monitoring protocols. Address each condition in your revised submission and resubmit it for final approval. This step is essential to demonstrate your commitment to principled research practices, especially when working with local ethics committees in Australia.
Rejection: If your application is denied, take the time to thoroughly analyze the reviewers' feedback. Identify the specific reasons for the rejection and consider how to address these concerns. This may involve revising your research design, improving ethical considerations, or providing supplementary information to clarify your proposal.
Follow-Up: Regardless of the outcome, maintaining open lines of communication is essential when working with local ethics committees in Australia. If you have questions about their feedback or need clarification on specific points, don’t hesitate to reach out. Engaging in dialogue can foster a better understanding of their expectations and enhance your chances of future success.
As C.S. Lewis wisely stated, "Integrity is doing the right thing, even when no one is watching." By effectively interpreting and responding to ethics review outcomes, you can ensure that your research aligns with ethical standards and is well-positioned for success in 2025.
Understanding the complexities of working with local ethics committees in Australia is crucial for any researcher looking to navigate the ethical landscape of human research effectively. By grasping the fundamental principles and regulations that govern these committees, researchers can ensure their proposals meet the necessary ethical standards, facilitating smoother interactions throughout the review process.
Key insights from the article underscore the importance of:
From selecting the right Human Research Ethics Committee (HREC) to responding appropriately to feedback, each step is vital in achieving ethical approval for research projects. Moreover, the emphasis on adhering to updated guidelines and fostering a cooperative relationship with ethics boards is paramount, as these elements significantly enhance the integrity of research practices.
Ultimately, engaging effectively with local ethics committees transcends mere compliance; it reflects a commitment to ethical research that respects the rights and welfare of human participants. Researchers are encouraged to approach this process with diligence and transparency, ensuring that their work not only adheres to regulatory standards but also contributes positively to the broader research community. By doing so, they can uphold the highest ethical standards and advance the field of human research in Australia.
What is the role of Human Research Ethics Committees (HRECs) in Australia?
HRECs evaluate research proposals involving human participants to ensure compliance with moral standards and protect the rights and welfare of participants.
Why is it important to understand the National Statement on Conduct in Human Research?
Familiarity with the National Statement is crucial because it outlines the principles and guidelines that researchers must adhere to when conducting research involving human participants.
What updates have been made to the National Statement in 2025?
The 2025 updates emphasize the necessity for compliance with international standards, including ICH Good Clinical Practice and ISO 14155, reinforcing the commitment to principled research practices.
What other regulations should researchers be aware of when working with ethics committees?
Researchers should review specific regulations set forth by the National Health and Medical Research Council (NHMRC) and any relevant state-specific guidelines to effectively organize their applications and discussions with review boards.
How do local ethics committees contribute to compliance in Australian clinical research?
Local ethics committees provide rigorous oversight, which contributes to notably high compliance rates with moral standards in Australian clinical research.
What is meant by HRECs being described as 'devolved regulators'?
This term suggests that HRECs have evolved within the broader regulatory landscape, but there is still a need for clear roles and responsibilities to enhance their effectiveness.
How can understanding the principles and regulations help in interactions with review boards?
A foundational understanding of these principles and regulations enables researchers to navigate the complexities of review board interactions and facilitate more seamless approval processes.