


This article delves into the critical FDA regulations that govern medical devices and their implications for clinical success. It underscores the necessity for manufacturers to comprehend and comply with regulations such as:
Such understanding is essential to ensure product safety, efficacy, and successful market entry. The significant impact of compliance on approval rates and market readiness of medical devices serves as compelling evidence of this necessity.
Navigating the intricate landscape of FDA regulations is a critical endeavor for manufacturers of medical devices. Understanding compliance can mean the difference between success and costly delays. This article delves into the essential regulations that govern medical devices, highlighting the importance of adhering to standards such as:
With nearly one-third of submissions facing initial rejection, the question looms: how can manufacturers effectively master these regulations to ensure their products not only meet compliance but also thrive in a competitive market?
The FDA supervises medical devices under the Federal Food, Drug, and Cosmetic Act, ensuring their safety and effectiveness for their intended applications. Key regulations include:
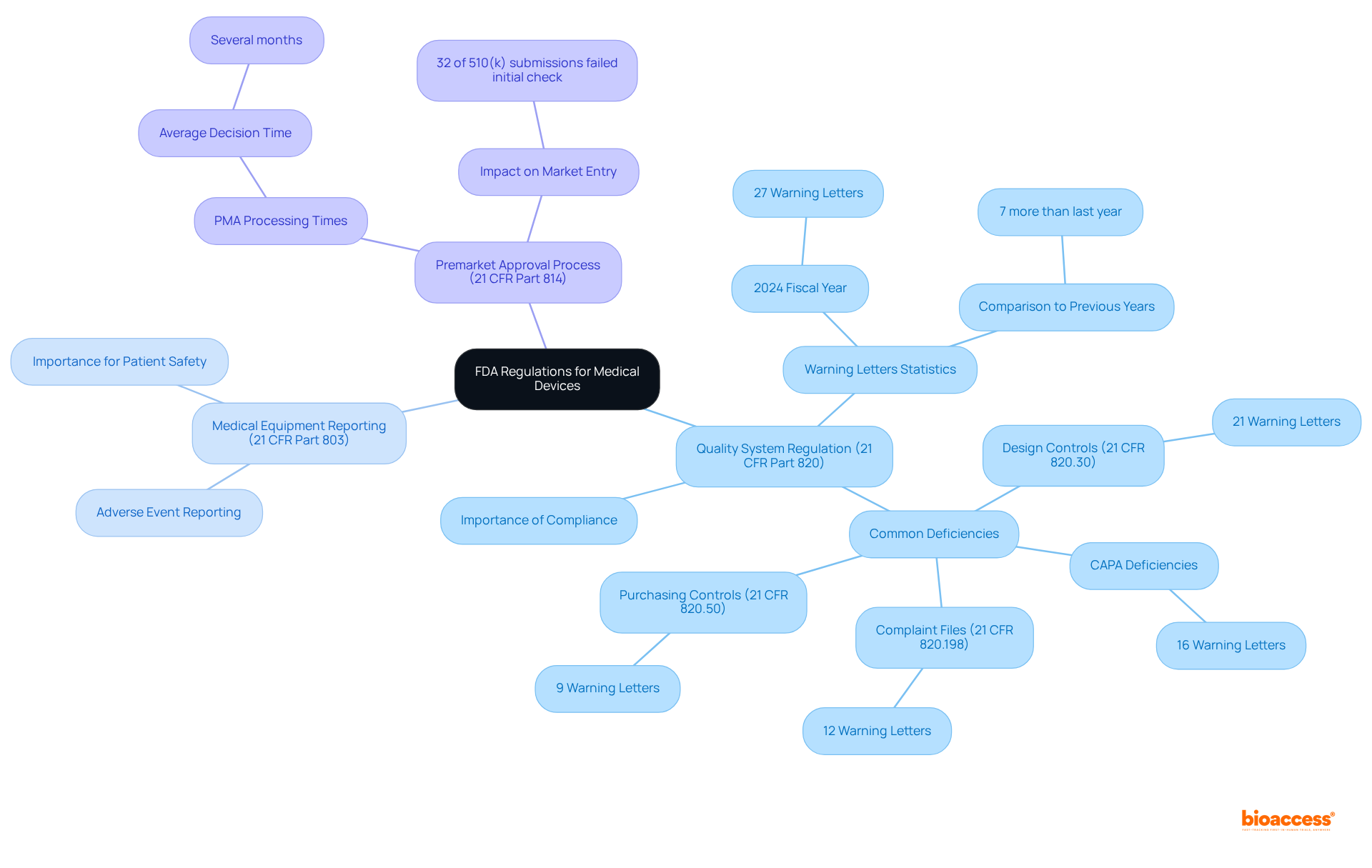
21 CFR Part 820: This regulation establishes the Quality System Regulation (QSR), requiring manufacturers to implement and maintain a robust quality system throughout the design and production phases of medical devices. Compliance with QSR is critical, as deficiencies in this area accounted for 27 Warning Letters in the 2024 fiscal year, highlighting the importance of rigorous quality management.
21 CFR Part 803: This regulation mandates Medical Equipment Reporting (MDR), obligating manufacturers to report adverse events and equipment defects promptly. Effective reporting is crucial for ensuring patient safety and adherence to regulations.
21 CFR Part 814: This section outlines the Premarket Approval (PMA) process for Class III products, representing the most stringent regulatory pathway. The average time to issue a decision on PMA applications can extend significantly, often taking several months, emphasizing the need for thorough preparation and adherence to FDA standards.
Navigating these regulations is essential for ensuring compliance and achieving successful results in the development of medical devices. With nearly 32% of FDA 510(k) submissions failing the initial acceptance for review check in recent years, down from 35% the previous year, understanding and adhering to these regulations can significantly impact a manufacturer's ability to bring products to market efficiently. As highlighted by industry specialists, "Complying with the Quality System Regulation is not merely a regulatory obligation; it is crucial for guaranteeing the safety and effectiveness of medical products in the market.
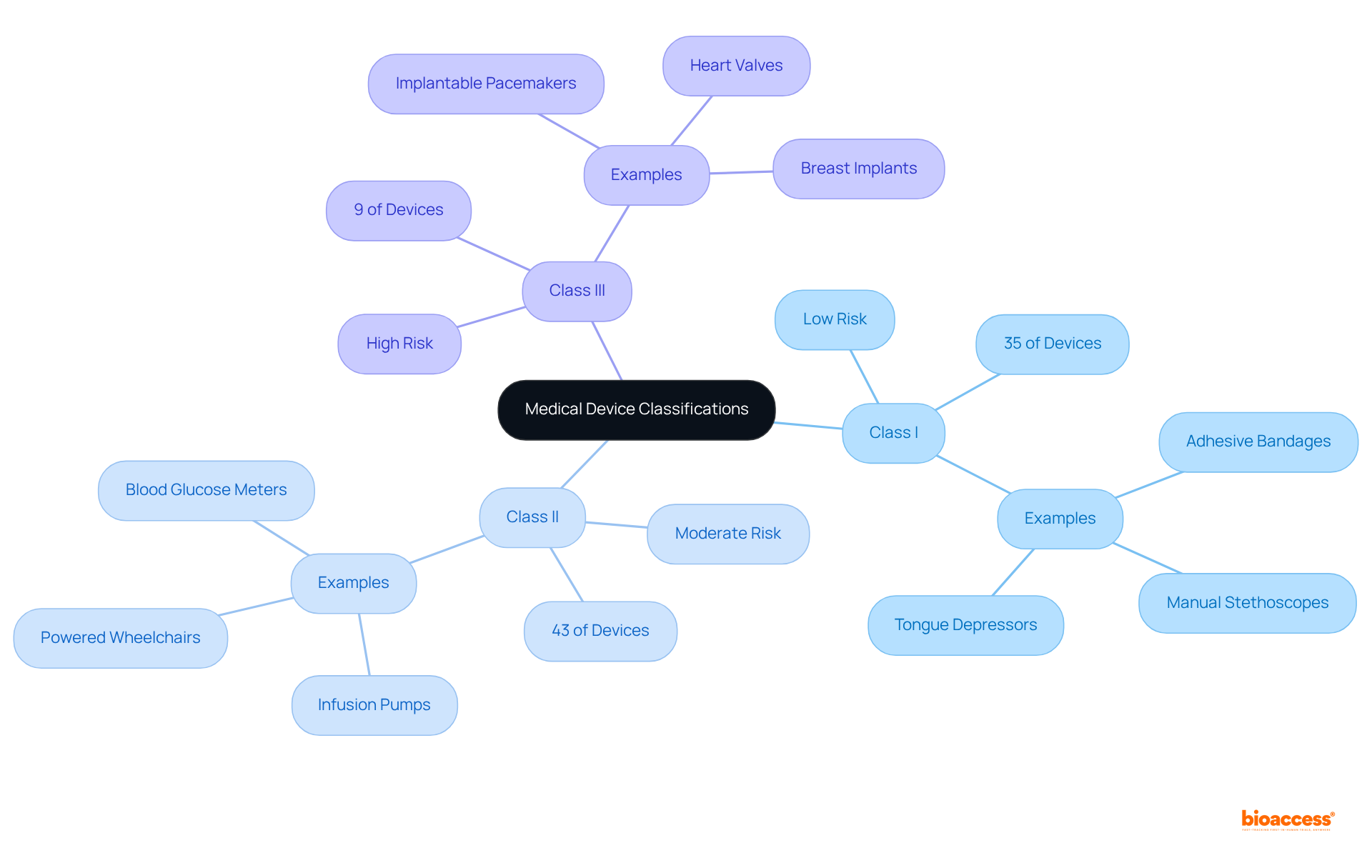
The FDA classifies medical devices into three primary categories based on their associated risk levels:
Class I: These low-risk devices typically do not require premarket notification. Approximately 35% of regulated medical instruments fall into this category, including simple items like adhesive bandages and manual stethoscopes. Their straightforward design often leads to minimal regulatory oversight, making them easier to bring to market. As Mike Drues, President of Vascular Sciences, points out, "Most class I products are exempt." While it's true that most class II items are non-exempt, there are exceptions in both categories.
Class II: Comprising approximately 43% of medical devices FDA, these items are regarded as moderate-risk and require a 510(k) premarket notification. This process mandates that manufacturers demonstrate their product's substantial equivalence to an already marketed item. Examples include infusion pumps and powered wheelchairs, which have more complex designs and necessitate additional regulatory controls to ensure safety and effectiveness. According to industry experts, "The essence of a definition of a medical instrument... is something, anything other than a drug that's intended to prevent, diagnose, or treat a disease, injury or condition."
Class III: Representing only 9% of approved medical devices FDA, Class III items are categorized as high-risk and necessitate Premarket Approval (PMA). This thorough procedure includes comprehensive scientific and compliance examination to verify the product's safety and effectiveness. Examples consist of implantable pacemakers and heart valves, which are essential for maintaining life and therefore encounter the most stringent compliance requirements. As emphasized by industry leaders, grasping these classifications is essential for producers of medical devices FDA, as they directly influence the compliance route, testing criteria, and overall market approach for product commercialization.
Understanding these classifications is crucial for producers, as they directly influence the regulatory pathway, testing requirements, and overall market strategy for product commercialization. Industry leaders stress the importance of effectively navigating these classifications to ensure compliance and facilitate successful market entry.
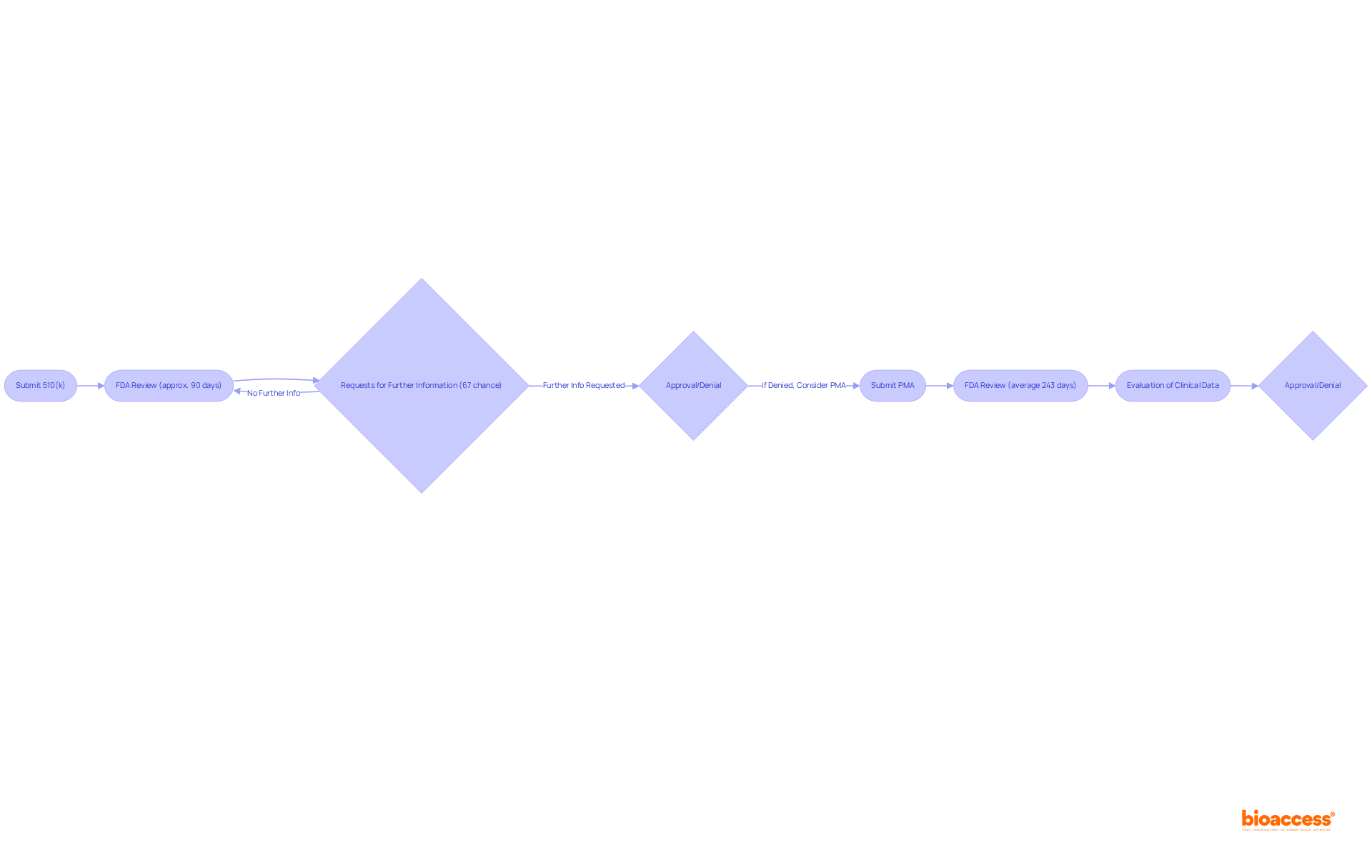
The premarket approval process for medical devices FDA is critical in the clinical research landscape and varies based on the device classification. For Class II devices, manufacturers must submit a 510(k) to demonstrate that their device is substantially equivalent to a predicate device. The FDA targets a review period of roughly 90 days; however, the actual average duration for approval has been reported at about 177 days, influenced by the intricacy of the product and the potential for requests for further information. Notably, nearly 67% of 510(k) submissions resulted in requests for further clarification during the substantive review process in the year up to September 2022, which can lead to delays and increased costs. Furthermore, 85% of FDA 510(k) applications received a Substantially Equivalent decision by September 2022, indicating a favorable success rate for submissions.
In contrast, Class III products undergo a more stringent Premarket Approval (PMA) process, requiring a thorough evaluation of clinical data to validate safety and effectiveness. The average time for PMA approval has recently been reported at 243 days, a significant decrease from 345 days prior to 2010, reflecting improvements in the process. However, this timeline can extend significantly based on the completeness of the submission and the necessity for additional information. Incomplete or poorly organized PMA applications can lead to delays or even denial by the FDA, emphasizing the need for meticulous preparation.
Both pathways necessitate thorough documentation, including detailed descriptions of the equipment, intended use, and clinical data, to meet the requirements set forth by medical devices FDA. Engaging with regulatory professionals early in the process is crucial, as their expertise can significantly enhance the likelihood of successful submissions and minimize potential delays.
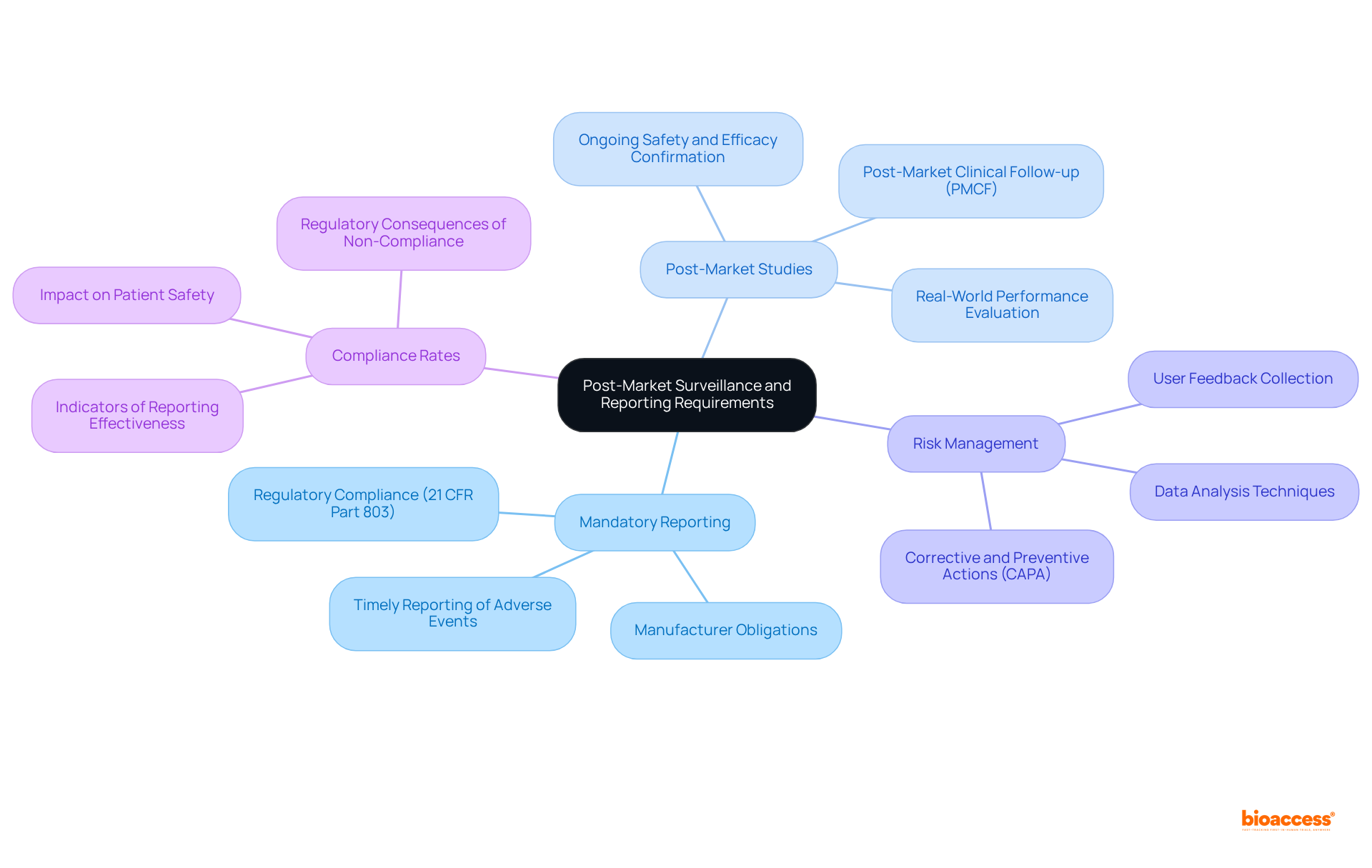
Post-market surveillance is essential for overseeing the safety and efficacy of medical instruments once they are in use. It is imperative that manufacturers engage in Mandatory Reporting, as they are required to inform the FDA about any adverse events or device defects associated with medical devices FDA under 21 CFR Part 803 within specified timeframes. This regulation mandates that producers notify the FDA when they ascertain that their products may have contributed to a death or serious injury, ensuring prompt measures to protect patient safety. Compliance rates under this regulation serve as critical indicators of the effectiveness of these reporting requirements.
Moreover, the FDA may mandate Post-Market Studies for medical devices to gather additional information regarding the product's performance in real-world environments. These studies are vital for confirming the ongoing safety and efficacy of medical instruments, allowing producers to proactively address any emerging concerns.
In addition, a robust Risk Management strategy is crucial for producers to identify and mitigate potential safety issues. This strategy must include organized methods for collecting user feedback and analyzing data, ensuring compliance with legal standards while enhancing product quality. As Felisiano Cipressi aptly noted, "An accurate understanding of the gathered data enables producers to make informed choices to enhance their product quality and to respond effectively to any inquiries from oversight bodies."
These requirements transcend mere regulatory obligations; they are foundational to maintaining the integrity of medical devices in the market and ensuring that corrective actions are implemented when necessary. As industry experts emphasize, effective post-market surveillance underscores a manufacturer's commitment to patient safety and innovation.
Navigating the complex landscape of FDA regulations for medical devices is crucial for ensuring the safety and effectiveness of these essential products. By understanding the regulatory framework—including the Quality System Regulation, Medical Device Reporting, and the Premarket Approval process—manufacturers can better position themselves for success in the competitive medical device market.
Key insights into the classification of medical devices reveal the varying levels of risk and regulatory scrutiny associated with each category:
Comprehending these distinctions is vital for manufacturers to strategize their market entry effectively. Additionally, the importance of thorough documentation and proactive engagement with regulatory professionals cannot be overstated, as these factors significantly influence the likelihood of successful submissions.
Ultimately, the commitment to post-market surveillance and adherence to reporting requirements reflects a manufacturer's dedication to patient safety and product quality. By prioritizing compliance and continuous improvement, industry leaders can not only fulfill regulatory obligations but also foster innovation and trust in the medical device sector. Embracing these principles will pave the way for a more effective and responsible approach to medical device development, ultimately benefiting patients and healthcare providers alike.
What is the role of the FDA in regulating medical devices?
The FDA supervises medical devices under the Federal Food, Drug, and Cosmetic Act, ensuring their safety and effectiveness for their intended applications.
What is the Quality System Regulation (QSR)?
The Quality System Regulation (QSR), established under 21 CFR Part 820, requires manufacturers to implement and maintain a robust quality system throughout the design and production phases of medical devices.
Why is compliance with the QSR important?
Compliance with the QSR is critical because deficiencies in this area accounted for 27 Warning Letters in the 2024 fiscal year, indicating the importance of rigorous quality management.
What does the Medical Equipment Reporting (MDR) regulation entail?
The Medical Equipment Reporting (MDR) regulation, outlined in 21 CFR Part 803, mandates manufacturers to report adverse events and equipment defects promptly to ensure patient safety and regulatory adherence.
What is the Premarket Approval (PMA) process?
The Premarket Approval (PMA) process, detailed in 21 CFR Part 814, is the most stringent regulatory pathway for Class III products, often taking several months for the FDA to issue a decision on PMA applications.
How does understanding FDA regulations impact medical device manufacturers?
Understanding and adhering to FDA regulations is essential for ensuring compliance and achieving successful results in the development of medical devices, as nearly 32% of FDA 510(k) submissions have failed the initial acceptance for review check in recent years.
What is the significance of complying with FDA regulations according to industry specialists?
Industry specialists emphasize that complying with the Quality System Regulation is not merely a regulatory obligation; it is crucial for guaranteeing the safety and effectiveness of medical products in the market.