


Mastering medical devices regulation is essential for clinical research success. This mastery ensures that devices are safe and effective prior to market introduction. This article outlines critical regulatory pathways, classifications, and strategies for conducting clinical trials. Understanding these regulations is paramount, as it facilitates successful compliance and market access, ultimately driving the success of clinical research initiatives.
Understanding the intricate landscape of medical device regulations is essential for clinical research professionals who aim to ensure safety and efficacy prior to market introduction. With oversight organizations such as the FDA and EMA setting the stage, this article explores the core principles and pathways that govern medical device compliance. It offers invaluable insights for navigating these complexities.
However, as regulations evolve and new challenges arise, researchers must consider:
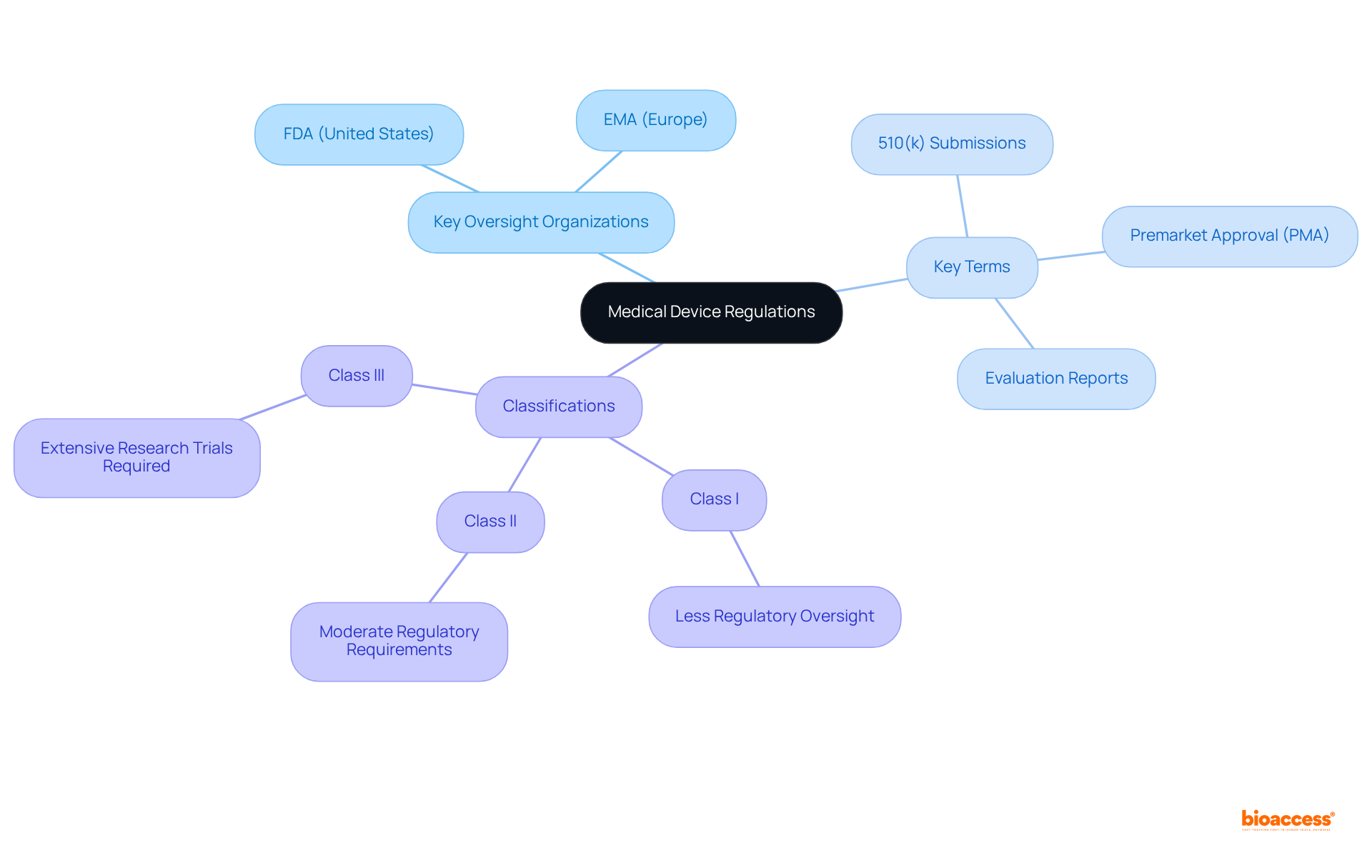
Medical devices regulation is crucial for ensuring the safety and efficacy of devices prior to their market introduction. Key oversight organizations, such as the FDA in the United States and the EMA in Europe, establish guidelines that manufacturers must follow. Understanding medical devices regulation necessitates familiarity with terms such as:
Each device is classified according to its risk level, which directly influences the compliance pathway it must navigate. For instance:
Grasping these classifications and their implications is vital for any clinical research professional aiming to effectively navigate the complexities of medical devices regulation.
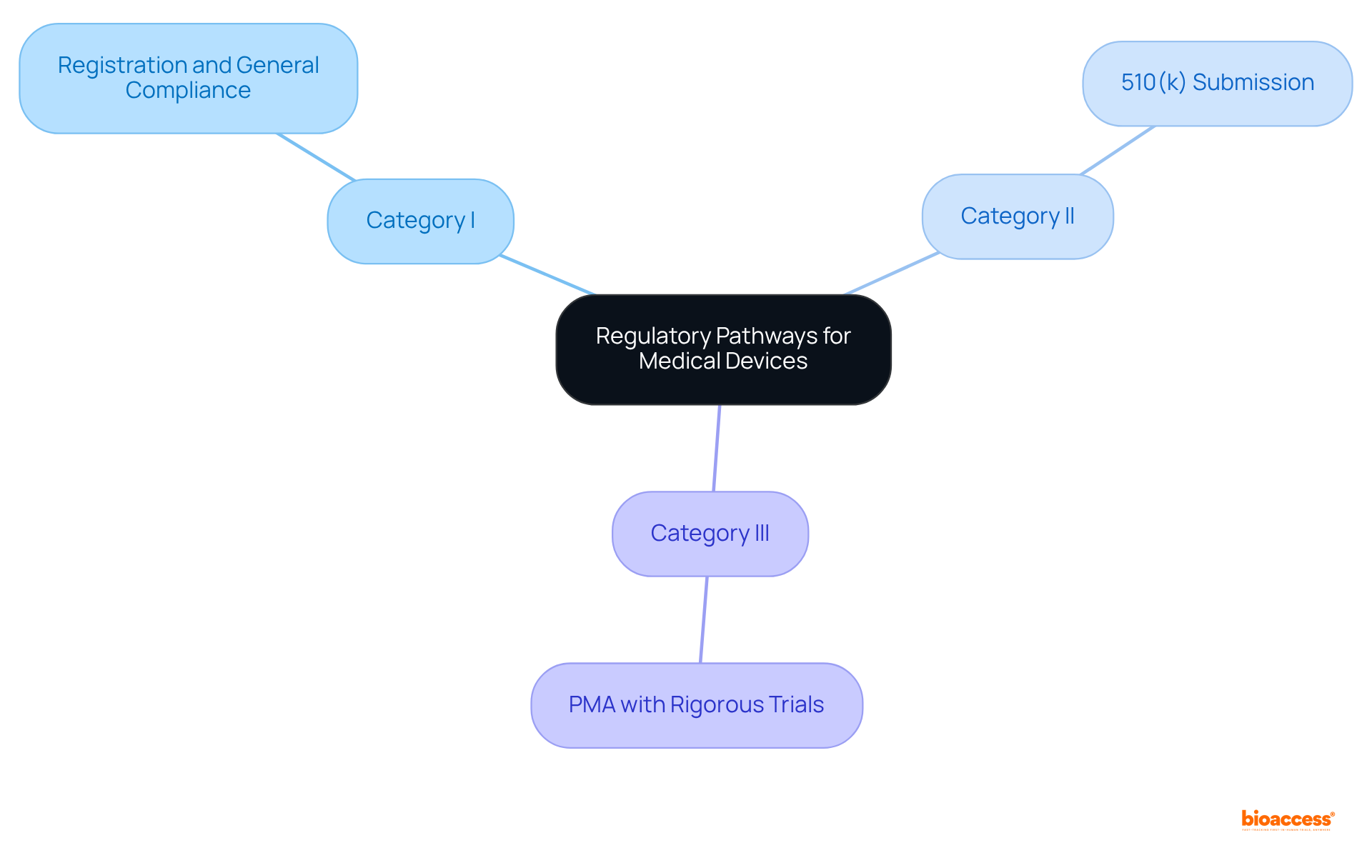
Regulatory pathways for medical devices regulation are crucial to understand, as they vary significantly based on classification. For instance:
Understanding these pathways is essential for research professionals, as it guides the planning of studies and adheres to medical devices regulation for the timeline of market introduction. Furthermore, researchers must remain vigilant about any regulatory changes that could impact their studies.
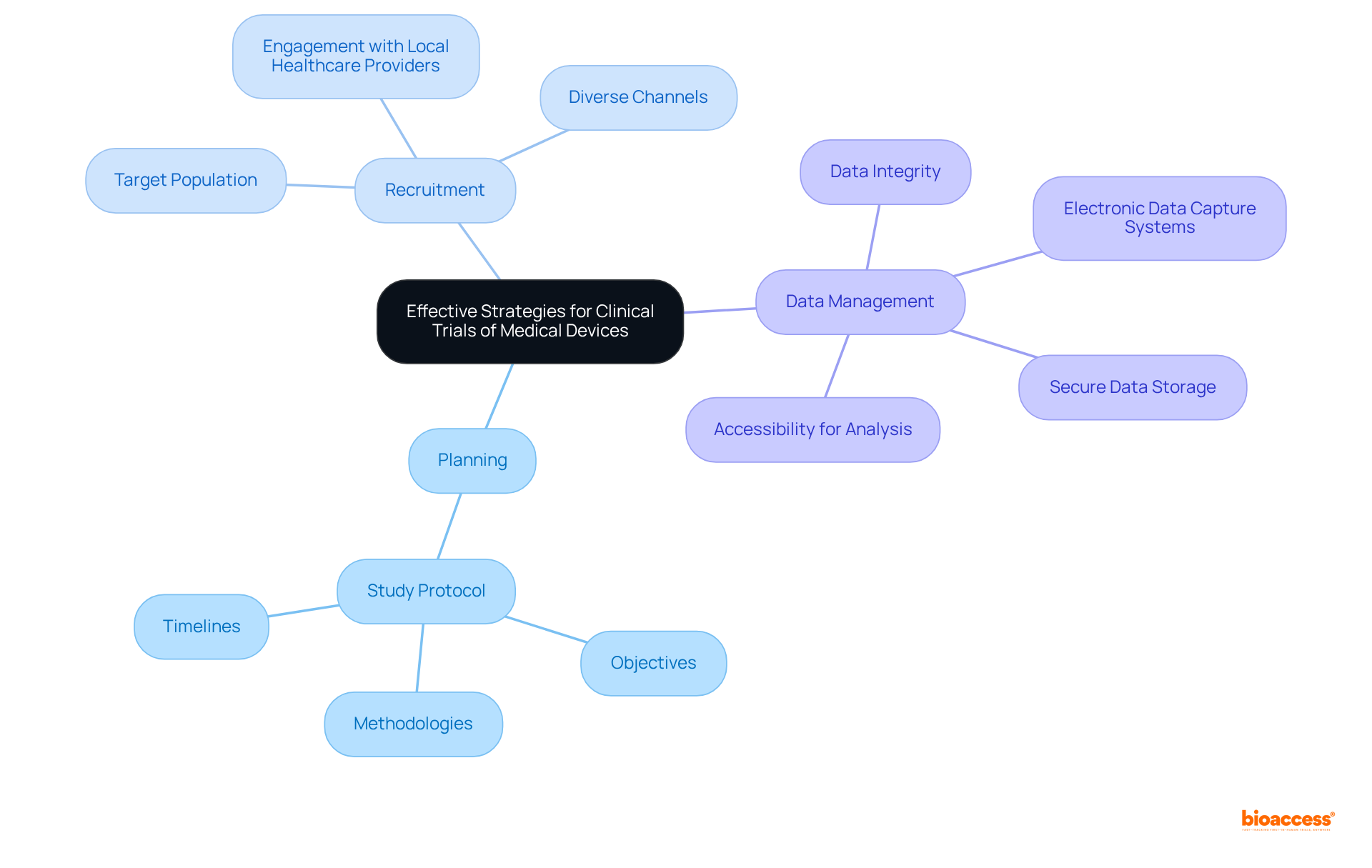
To implement effective strategies for research trials of medical devices, researchers must concentrate on several key areas:
A well-structured study protocol is essential; it should detail objectives, methodologies, and timelines. Recruitment strategies must be tailored to the target population, utilizing diverse channels to ensure a representative sample. Engaging with local healthcare providers can significantly facilitate participant enrollment.
Furthermore, robust data management practices are crucial for upholding data integrity and adhering to legal requirements. This includes the use of electronic data capture systems and ensuring that all data is securely stored and easily accessible for analysis.
By focusing on these areas, researchers can enhance the quality and reliability of their clinical trials, ultimately leading to successful regulatory submissions that comply with medical devices regulation and market access.
Mastering the intricacies of medical device regulation is essential for professionals in clinical research, as these regulations ensure that devices are both safe and effective before they reach the market. Understanding the various classifications and regulatory pathways, from Class I to Class III devices, is crucial for navigating the compliance landscape. This knowledge empowers researchers to strategically plan their studies and adhere to the necessary guidelines, ultimately facilitating a smoother path to market introduction.
Key insights discussed throughout this article highlight the importance of:
These elements serve as the foundation for conducting successful clinical trials that meet regulatory standards. By focusing on these areas, researchers can significantly enhance the quality and reliability of their trials, leading to favorable outcomes in regulatory submissions.
In conclusion, the significance of medical device regulation in clinical research cannot be overstated. As the landscape evolves, staying informed about current trends and best practices is vital for success. By prioritizing compliance and embracing effective strategies, researchers can contribute to the advancement of medical devices that ultimately improve patient care and safety.
Why is medical device regulation important?
Medical device regulation is crucial for ensuring the safety and efficacy of devices before they are introduced to the market.
Which organizations oversee medical device regulations?
Key oversight organizations include the FDA (Food and Drug Administration) in the United States and the EMA (European Medicines Agency) in Europe.
What are some key terms associated with medical device regulation?
Important terms include premarket approval (PMA), 510(k) submissions, and evaluation reports.
How are medical devices classified?
Medical devices are classified according to their risk level, which influences the compliance pathway they must follow.
What is the regulatory oversight for Class I products?
Class I products typically require less regulatory oversight compared to higher-risk classes.
What is required for Class III products?
Class III products may necessitate extensive research trials due to their higher risk level.
Why is it important for clinical research professionals to understand medical device regulations?
Understanding these regulations and classifications is vital for effectively navigating the complexities of medical device regulation.