


Phase 3 medical trials are crucial for validating the efficacy and safety of new treatments prior to their market approval. These trials involve extensive participant testing and rigorous regulatory oversight, ensuring that new therapies not only demonstrate significant advantages over existing options but also adhere to ethical standards. This includes:
These elements are vital for guaranteeing patient protection and achieving successful drug approval outcomes.
The landscape of medical innovation hinges on the success of Phase 3 clinical trials, the final proving ground for new therapies before they can reach the market. These trials, involving extensive participant groups and rigorous methodologies, are meticulously designed to validate the efficacy and safety of treatments while adhering to strict ethical standards.
With billions of dollars at stake and the potential to transform patient care, stakeholders face the critical question: how can they navigate the complexities and challenges inherent in these pivotal studies?
Phase 3 medical trials represent the final testing stage prior to submitting a new medication or procedure for regulatory approval. Typically involving several hundred to thousands of participants, these studies are meticulously designed to validate the intervention's efficacy while closely monitoring potential side effects. A primary objective is to assess the new approach against the existing standard of care, ensuring it offers a significant advantage to patients.
The data generated from Phase 3 studies is critical, as it forms the foundation for the drug's labeling and marketing authorization. Successful outcomes from these trials are essential; without them, a treatment cannot advance to market, underscoring their vital role in fostering medical innovation and enhancing healthcare. Furthermore, the rigorous nature of these assessments, often lasting from one to four years, underscores the commitment to patient safety and the thorough evaluation of new therapies.
Therefore, Phase 3 studies are not only pivotal in determining a drug's market entry but also in shaping the future of healthcare by ensuring that only safe and effective treatments reach patients.
At bioaccess, we offer comprehensive clinical study management services, including:
Our expertise ensures that these trials are conducted efficiently and in compliance with regulatory standards, which is crucial for the success of medical device startups navigating the complexities of clinical research. Favorable results from the phase 3 medical trials significantly improve the likelihood of drug approval and market launch, emphasizing their importance in advancing medical progress and enhancing healthcare outcomes.
Additionally, these studies operate under stringent regulatory oversight to ensure participant safety, ethical practices, and scientific integrity. Without successful phase 3 medical trials, a remedy cannot progress to market, highlighting their essential role in promoting medical innovation and enhancing patient care.
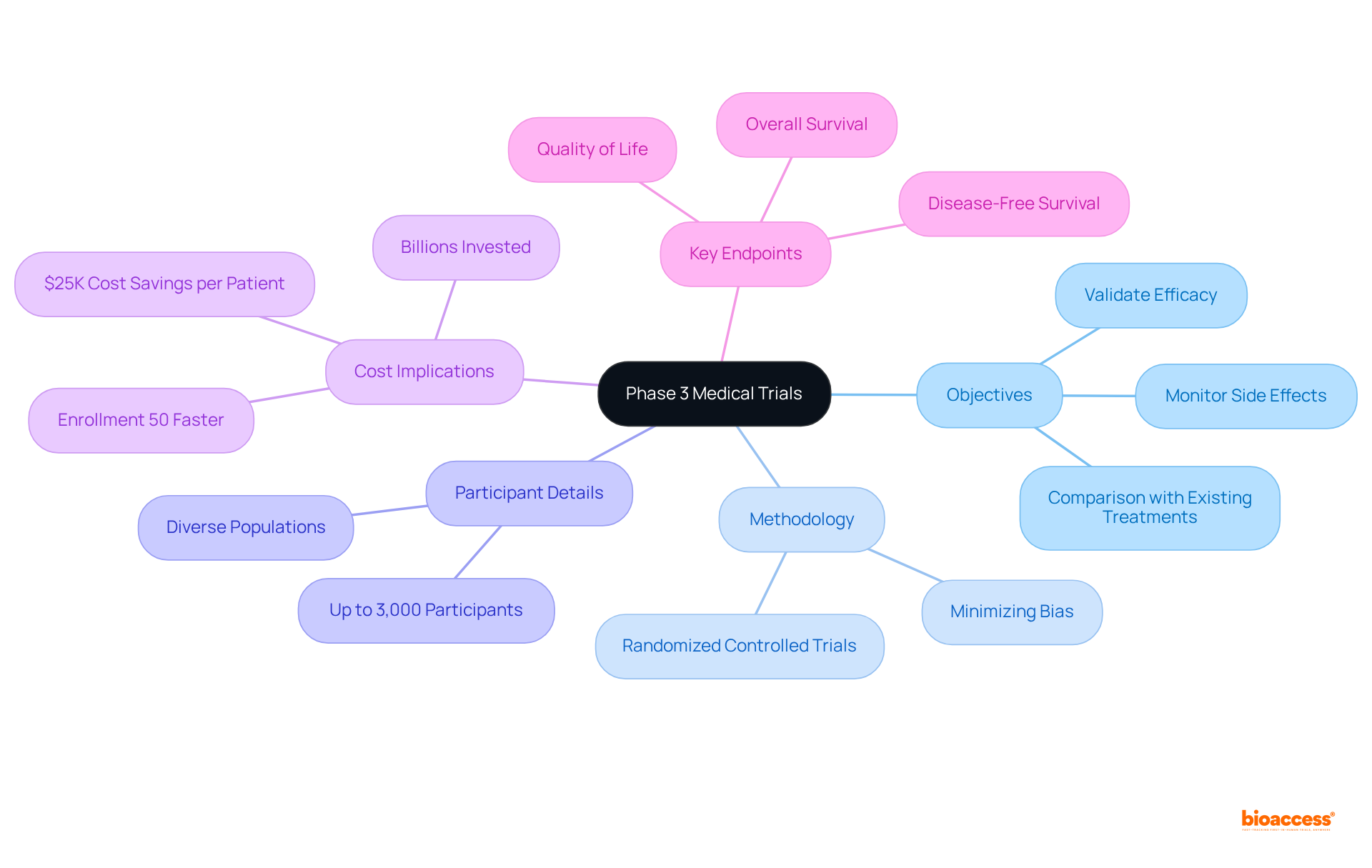
The objectives of phase 3 medical trials are crucial, primarily aimed at validating the efficacy of new therapies, monitoring side effects, and comparing them with existing treatments. These studies typically employ randomized controlled trials (RCTs), recognized as the gold standard in clinical research. In RCTs, participants are randomly assigned to receive either the new treatment or a placebo/control, effectively minimizing bias and ensuring that observed outcomes can be directly attributed to the intervention itself.
With bioaccess®, these studies can be accelerated, achieving participant enrollment 50% faster than traditional Western sites. This efficiency leads to substantial cost savings, estimated at $25K per patient, due to FDA-ready data that eliminates rework and delays.
Phase 3 studies frequently involve as many as 3,000 participants, enhancing the diversity and applicability of results across various populations. Key endpoints, such as overall survival, disease-free survival, and quality of life, are clearly defined to provide a thorough assessment of the intervention's impact. This structured methodology not only bolsters the robustness of the data collected but also ensures adherence to regulatory scrutiny, ultimately facilitating the successful advancement of innovative therapies to market.
As Dr. Ananya Mandal articulates, "The primary emphasis of phase 3 medical trials is to showcase and validate the initial evidence collected in the earlier assessments that the medication is a safe, advantageous, and effective remedy for the intended purpose." Furthermore, it is essential to recognize that billions may be invested in propelling a drug through phase 3 medical trials, underscoring the financial risks associated with these studies.
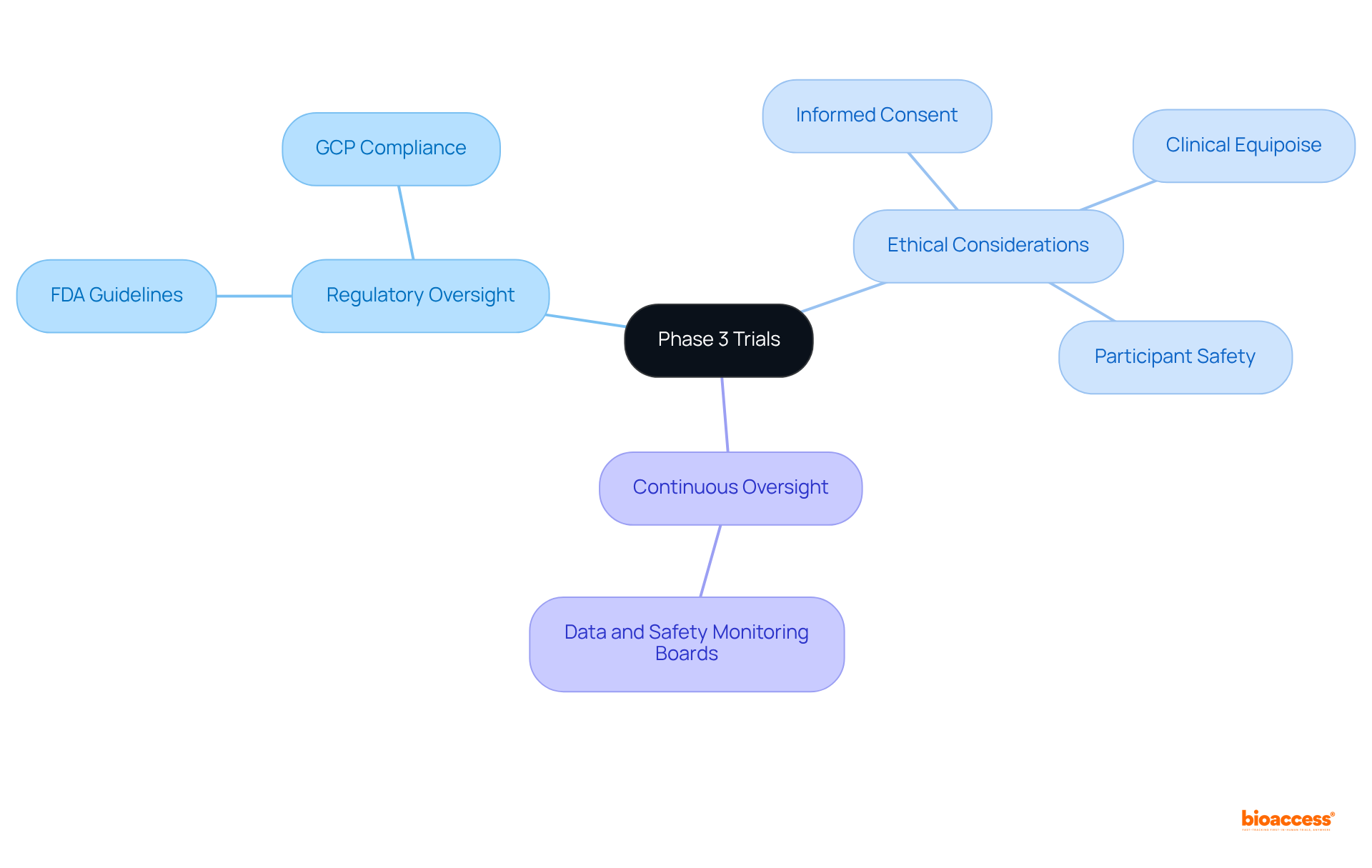
Phase 3 studies are governed by rigorous oversight to ensure ethical and safe practices. Regulatory agencies, such as the FDA, mandate comprehensive documentation and adherence to Good Clinical Practice (GCP) guidelines throughout the study process. A pivotal ethical consideration is informed consent, which ensures that participants are thoroughly informed of the risks and benefits prior to enrollment. This process typically involves detailed discussions and written documentation, guaranteeing clarity and understanding.
Moreover, the principle of clinical equipoise must be upheld, signifying that there exists genuine uncertainty within the medical community regarding the comparative therapeutic merits of each study group. This ethical framework is essential for safeguarding participants and upholding the integrity of the research.
Continuous oversight by independent Data and Safety Monitoring Boards (DSMBs) is equally vital, as they assess treatment safety and provide recommendations on study continuation based on interim results.
Statistics indicate that approximately 58%-65% of drugs entering Phase 3 trials ultimately secure FDA approval, highlighting the significance of these ethical considerations in achieving successful outcomes.
Phase 3 clinical trials are pivotal in the drug development process, marking the final testing phase prior to regulatory approval for a treatment. These trials are instrumental in validating the efficacy and safety of new therapies, ensuring that they deliver significant benefits over existing options. The extensive data gathered during this phase is essential for propelling medical innovation and enhancing patient care.
This article underscores critical elements of Phase 3 trials, including their objectives, methodologies, and ethical considerations. By utilizing randomized controlled trials (RCTs), researchers effectively minimize bias, leading to trustworthy outcomes. The considerable financial investments required underscore the complexity of this stage, while strict adherence to ethical practices—such as informed consent and safety monitoring—upholds the integrity of the research.
In summary, Phase 3 clinical trials are crucial in shaping the future of healthcare. With the increasing demand for innovative therapies, it is vital to champion the rigorous processes that ensure safe and effective treatments for patients. Acknowledging the significance of these trials cultivates understanding among stakeholders and strengthens the commitment to advancing medical science and improving patient outcomes.
What are Phase 3 clinical trials?
Phase 3 clinical trials are the final testing stage for a new medication or procedure before regulatory approval. They typically involve several hundred to thousands of participants and are designed to validate the efficacy of the intervention while monitoring potential side effects.
Why are Phase 3 clinical trials important?
Phase 3 trials are crucial because they provide the data needed for a drug's labeling and marketing authorization. Successful outcomes are essential for a treatment to advance to market, thereby fostering medical innovation and enhancing healthcare.
How long do Phase 3 clinical trials typically last?
Phase 3 clinical trials often last from one to four years, reflecting the rigorous nature of the assessments conducted to ensure patient safety and the thorough evaluation of new therapies.
What is the primary objective of Phase 3 clinical trials?
The primary objective of Phase 3 trials is to assess the new treatment against the existing standard of care, ensuring it offers a significant advantage to patients.
What services does bioaccess offer for clinical study management?
Bioaccess offers comprehensive clinical study management services, including feasibility studies, site selection, compliance reviews, study setup, import permits, project management, and reporting on serious and non-serious adverse events.
How do Phase 3 trials impact drug approval and market launch?
Favorable results from Phase 3 clinical trials significantly improve the likelihood of drug approval and market launch, emphasizing their importance in advancing medical progress and enhancing healthcare outcomes.
What oversight do Phase 3 clinical trials operate under?
Phase 3 clinical trials operate under stringent regulatory oversight to ensure participant safety, ethical practices, and scientific integrity.