


Phase III clinical studies are pivotal in validating the efficacy and safety of new treatments. They provide the definitive evidence required for regulatory approval and the establishment of new standards of care. The methodologies, regulatory considerations, and innovative recruitment strategies detailed in the article underscore their importance. These elements contribute to timely and successful study outcomes, ultimately enhancing patient care and expanding medical knowledge. Through a comprehensive understanding of these studies, stakeholders can navigate the complexities of clinical research more effectively.
Phase III clinical studies represent a pivotal moment in the drug development process, characterized by high stakes and the potential for significant medical advancements. These studies are meticulously crafted to evaluate the efficacy and safety of new treatments, typically engaging large and diverse patient populations to guarantee that the findings are relevant to the broader public.
Nevertheless, the path to successful Phase III trials is laden with challenges, including stringent regulatory demands and recruitment obstacles that can impede progress.
What strategies can researchers implement to navigate these complexities and ensure that new therapies are delivered to the patients who need them the most?
Phase III clinical studies play a pivotal role in the drug development continuum, meticulously designed to validate the efficacy and safety of new treatments against established therapies. Typically involving large patient groups across diverse locations, these studies ensure a comprehensive representation, which is vital for the generalizability of results. The primary objective is to collect robust data that will support regulatory approval and enhance clinical practice.
The significance of Stage III studies is profound; they provide the definitive evidence required to establish a new treatment as a standard of care, thereby improving patient outcomes and expanding medical knowledge. For example, successful phase III clinical studies have led to the approval of innovative cancer treatments that significantly enhance survival rates compared to existing alternatives.
Furthermore, advancements in study design and patient recruitment methods, such as bioaccess®'s ability to secure ethical approvals in just 4-6 weeks and guarantee enrollment that is 50% quicker than conventional markets, have expedited the process, facilitating faster access to potentially life-saving therapies. Notably, bioaccess® also delivers substantial cost reductions of $25K per patient, making clinical studies more efficient and accessible.
The impact of these studies extends beyond individual patient benefits, influencing medication approval rates and shaping future research trajectories. As highlighted by the American Cancer Society medical and editorial content team, the objective of this phase III clinical studies stage is to assist in accelerating the drug approval process, underscoring the essential role of phase III clinical studies in fostering medical innovation.
Phase III clinical studies play a pivotal role in clinical research, primarily aimed at evaluating the efficacy of new therapies, monitoring side effects, and comparing them with existing standard treatments. Typically, these studies employ randomized controlled designs, where participants are randomly assigned to receive either the new treatment or a control, such as a placebo or standard treatment. This approach significantly reduces bias and enhances the reliability of the findings.
Moreover, adaptive designs may be implemented, allowing for adjustments based on interim results, which can optimize resource allocation and ultimately improve patient outcomes. For example, phase III clinical studies assessing a new diabetes medication might not only evaluate its effectiveness against the current standard treatment but also examine long-term safety and quality of life metrics. Such comprehensive methodologies ensure that the trials produce robust data essential for regulatory approval and subsequent market success.
Additionally, bioaccess® streamlines the process of obtaining ethical approvals in just 4-6 weeks and achieves patient enrollment 50% faster than traditional markets, resulting in savings of $25K per patient with FDA-ready data—these efficient processes guarantee timely results. These distinct advantages highlight bioaccess®'s leadership in facilitating medical device clinical trials across Latin America.
Phase III clinical studies are subject to stringent oversight, ensuring the safety of volunteers and the integrity of data. Regulatory bodies such as the FDA and EMA enforce adherence to Good Clinical Practice (GCP) guidelines, which encompass vital ethical considerations, including:
A fundamental ethical principle, maintaining clinical equipoise, ensures genuine uncertainty about the superiority of treatments. This framework is crucial for justifying the randomization of subjects and the use of placebo controls when appropriate.
For example, a Stage III study evaluating a new cardiovascular medication must convincingly demonstrate that the anticipated benefits significantly outweigh the associated risks, while also ensuring that participants are fully informed about their roles and the implications of the study. Notably, approximately 50%-60% of medications entering phase III clinical studies ultimately receive FDA approval, highlighting the importance of these ethical guidelines in fostering successful outcomes in clinical research.
Moreover, challenges such as recruitment difficulties can substantially impact the success of phase III clinical studies, highlighting the need for effective strategies to engage participants. As bioaccess® emphasizes, their expertise in clinical study management—including site feasibility and investigator selection—along with their accelerated patient recruitment and site activation services, can effectively address these challenges. By leveraging bioaccess®'s comprehensive approach, clinical researchers can enhance the likelihood of achieving successful study results.
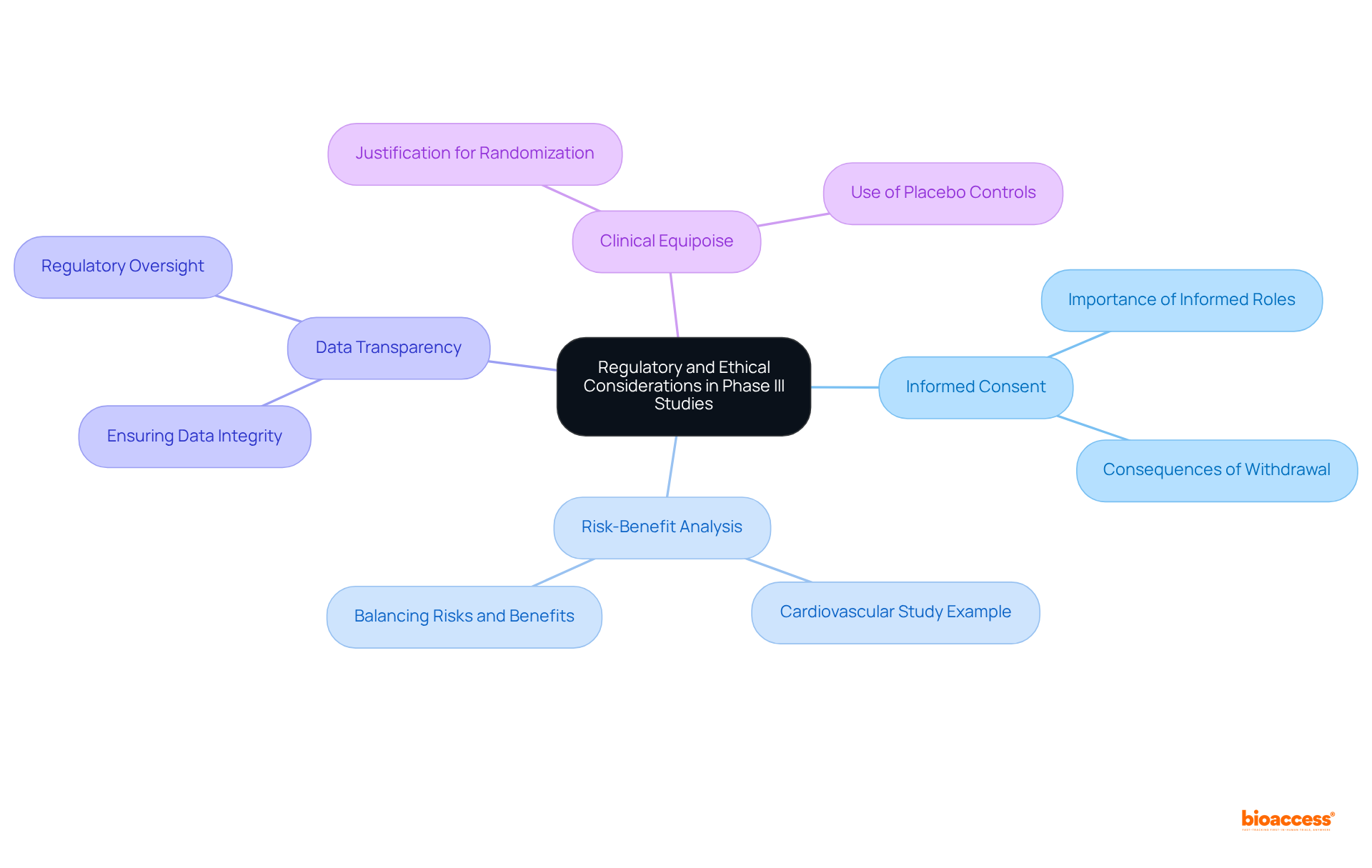
The recruitment and retention of subjects in phase III clinical studies present formidable challenges that can result in significant delays or even failures of the studies. Key barriers include:
Alarmingly, over 80% of studies fail to enroll on time, underscoring the urgency of addressing these recruitment challenges. To effectively tackle these issues, researchers can adopt targeted recruitment strategies, including:
Notably, collaborations such as that of GlobalCare Clinical Studies and bioaccess™ have demonstrated the capacity to improve clinical study ambulatory services in Colombia, achieving over a 50% reduction in recruitment time and a retention rate exceeding 95%. This partnership illustrates how leveraging local knowledge can significantly enhance recruitment and retention initiatives in Latin America, particularly by addressing the logistical obstacles faced by participants.
Improving attendee retention is equally critical and can be achieved through:
For instance, a Phase III study examining a new asthma treatment could incorporate telehealth consultations to alleviate travel challenges, thereby enhancing participant retention and the overall success of the research. Research indicates that regular interaction with participants substantially boosts retention rates, with 88% of dropouts attributed to missed follow-ups, non-compliance with protocol, and withdrawal of consent. The demeanor and communication skills of research personnel are essential in fostering a supportive environment, which is vital for maintaining participant interest and commitment throughout the study period.
Furthermore, delays in hiring can lead to considerable financial repercussions for pharmaceutical firms, with potential losses ranging from $600,000 to $8 million for each day of testing delays. By addressing these challenges with innovative strategies, including successful partnerships like that of GlobalCare Clinical Trials and bioaccess™, researchers can enhance the effectiveness of phase III clinical studies and ensure timely completion.
Phase III clinical studies are pivotal in the drug development landscape, serving as the definitive stage to validate new treatments against established therapies. These studies not only provide crucial evidence for regulatory approval but also enhance clinical practice, ultimately leading to improved patient outcomes. By employing robust methodologies and innovative recruitment strategies, such as those offered by bioaccess®, the efficiency and accessibility of these trials have significantly increased, ensuring the timely delivery of potentially life-saving therapies.
Key insights from this exploration reveal the multifaceted nature of Phase III studies, encompassing rigorous methodologies, ethical considerations, and the challenges of participant recruitment and retention. The emphasis on randomized controlled designs and adaptive methodologies ensures that the data collected is reliable and meaningful. Furthermore, understanding the regulatory environment and ethical frameworks surrounding these studies is vital for their success, as it fosters trust and safety among participants.
In light of the critical role that Phase III clinical studies play in advancing medical innovation, it is imperative for stakeholders to prioritize effective recruitment and retention strategies. Engaging communities, leveraging technology, and fostering supportive environments can significantly enhance participation rates. By proactively addressing these challenges, the medical community can continue to make strides in drug development, ultimately transforming patient care and expanding therapeutic options.
What are Phase III clinical studies?
Phase III clinical studies are pivotal trials in the drug development process designed to validate the efficacy and safety of new treatments compared to established therapies. They typically involve large patient groups across diverse locations.
Why are Phase III clinical studies important?
They provide definitive evidence required to establish a new treatment as a standard of care, which improves patient outcomes and expands medical knowledge. Successful studies have led to the approval of innovative treatments that significantly enhance survival rates.
How do Phase III studies contribute to regulatory approval?
The primary objective of Phase III studies is to collect robust data that supports regulatory approval, ensuring that new treatments are safe and effective before they are made available to the public.
What advancements have been made in Phase III study design and patient recruitment?
Advancements include faster ethical approvals, which can be secured in just 4-6 weeks, and quicker patient enrollment, which is 50% faster than conventional methods, facilitating faster access to potentially life-saving therapies.
What cost benefits are associated with Phase III clinical studies?
Bioaccess® offers substantial cost reductions of $25,000 per patient, making clinical studies more efficient and accessible.
How do Phase III studies influence medication approval rates and future research?
The impact of Phase III studies extends beyond individual patient benefits; they influence medication approval rates and help shape the direction of future research, thereby fostering medical innovation.