


The article addresses the mastery of clinical imaging endpoints in radiopharmaceuticals, a critical factor for achieving successful outcomes in medical research and treatment. It underscores the necessity of grasping essential endpoints such as:
Moreover, it highlights the significance of adhering to regulatory standards and implementing effective patient recruitment strategies. These elements are vital for enhancing the reliability and applicability of radiopharmaceutical studies within clinical environments.
Radiopharmaceuticals are revolutionizing the landscape of medical imaging, providing unparalleled insights into physiological processes that significantly enhance diagnostic precision.
With the market for these specialized agents projected to grow substantially, it becomes crucial for researchers to grasp the key clinical imaging endpoints, as this understanding is essential for translating findings into tangible patient benefits.
However, navigating the complexities of regulatory compliance, ethical considerations, and effective patient recruitment presents formidable challenges.
What strategies can be employed to master these clinical imaging endpoints and ensure the success of radiopharmaceutical studies?
Radiopharmaceuticals are specialized agents that incorporate radioactive isotopes, serving both diagnostic and therapeutic functions in medicine. Their primary role in medical imaging is to provide intricate insights into physiological processes, enabling healthcare professionals to diagnose diseases with precision. Notably, Technetium-99m stands as a cornerstone in imaging modalities such as SPECT (Single Photon Emission Computed Tomography), where it is utilized to assess organ function and identify abnormalities.
In 2025, Technetium-99m is projected to dominate SPECT imaging, underscoring its significance in the field. Understanding the pharmacokinetics and biodistribution of these agents is crucial for designing effective clinical trials and accurately interpreting radiopharma clinical imaging endpoints. This knowledge not only enhances the effectiveness of radioactive drugs but also propels the advancement of personalized medicine, where targeted therapies can be customized to individual patient needs.
Moreover, the market for radioactive pharmaceuticals is anticipated to expand at a compound annual growth rate (CAGR) of 16.4% from 2024 to 2029, with forecasts suggesting a market size of USD 19.6 billion by 2034. As Sandeep Singh Negi states, 'Radiopharmaceuticals represent a cutting-edge frontier in modern medicine, offering highly targeted diagnostic and therapeutic solutions, particularly in oncology, cardiology, and neurology.' However, challenges such as the short half-lives of isotopes and high production costs remain significant hurdles in the field.
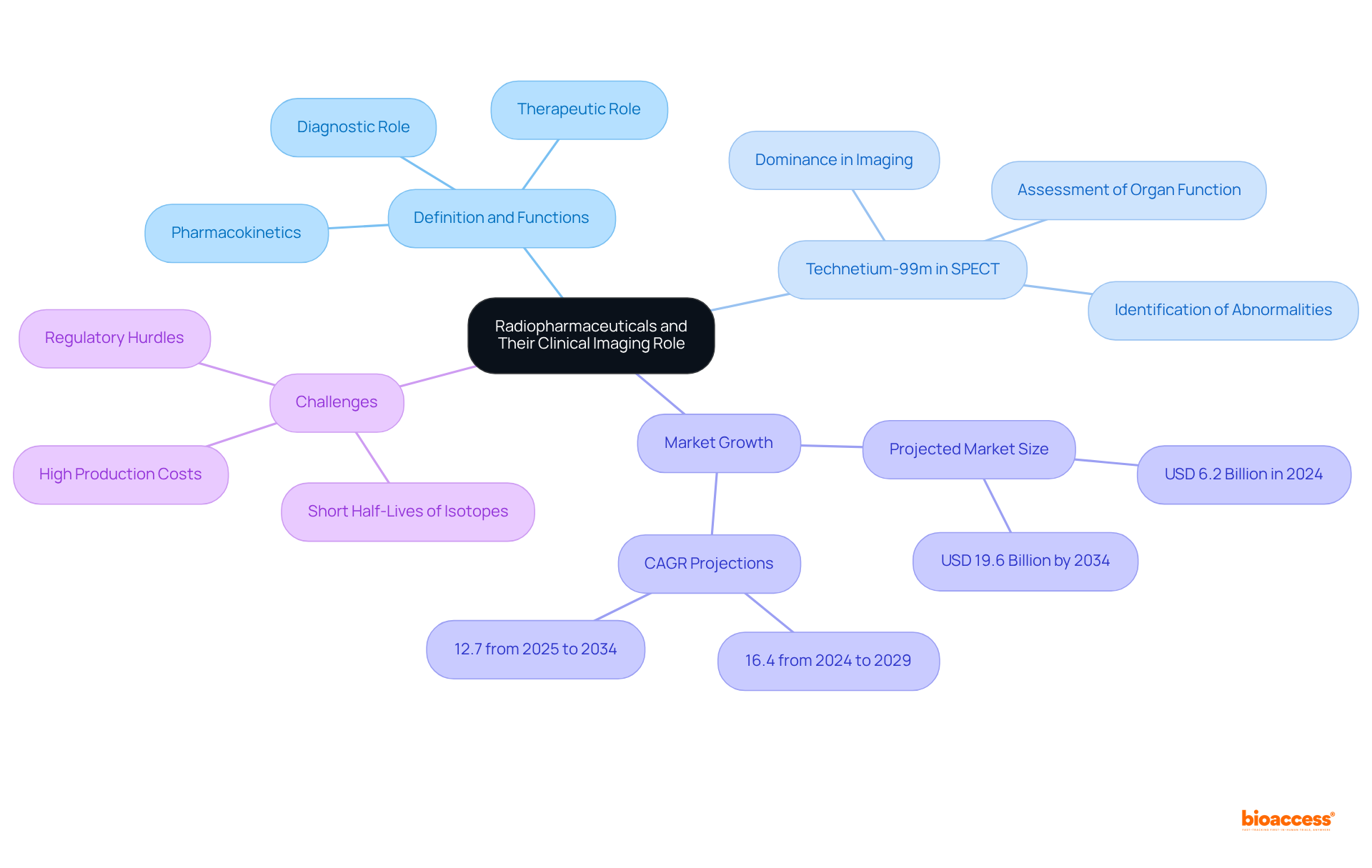
In radiopharmaceutical research, critical radiopharma clinical imaging endpoints encompass:
All of which are vital for assessing treatment effectiveness. The Response Evaluation Criteria in Solid Tumors (RECIST) is widely utilized in oncology studies to gauge tumor reduction or expansion, providing a standardized method for evaluating treatment outcomes. For example, RECIST defines progression as a 20% increase in the sum of target lesion diameters, ensuring consistency in reporting across various studies.
Furthermore, the integration of quantitative imaging biomarkers enhances the objectivity of treatment response evaluations, thereby reinforcing the reliability of study results. Choosing scientifically valid and clinically relevant radiopharma clinical imaging endpoints is crucial for translating findings into practical applications, ultimately enhancing patient outcomes in the realm of radiopharmaceuticals.
Notably, the ALSYMPCA study reported a median overall survival of 14.9 months for patients treated with radium-223, highlighting the efficacy of RECIST criteria in evaluating treatment results. Additionally, oncologists emphasize that aligning PFS definitions and measurements between observational studies and randomized controlled trials is essential for ensuring the reliability of evidence in oncology. Incorporating these elements will bolster the dependability of studies and their applicability in real-world settings.
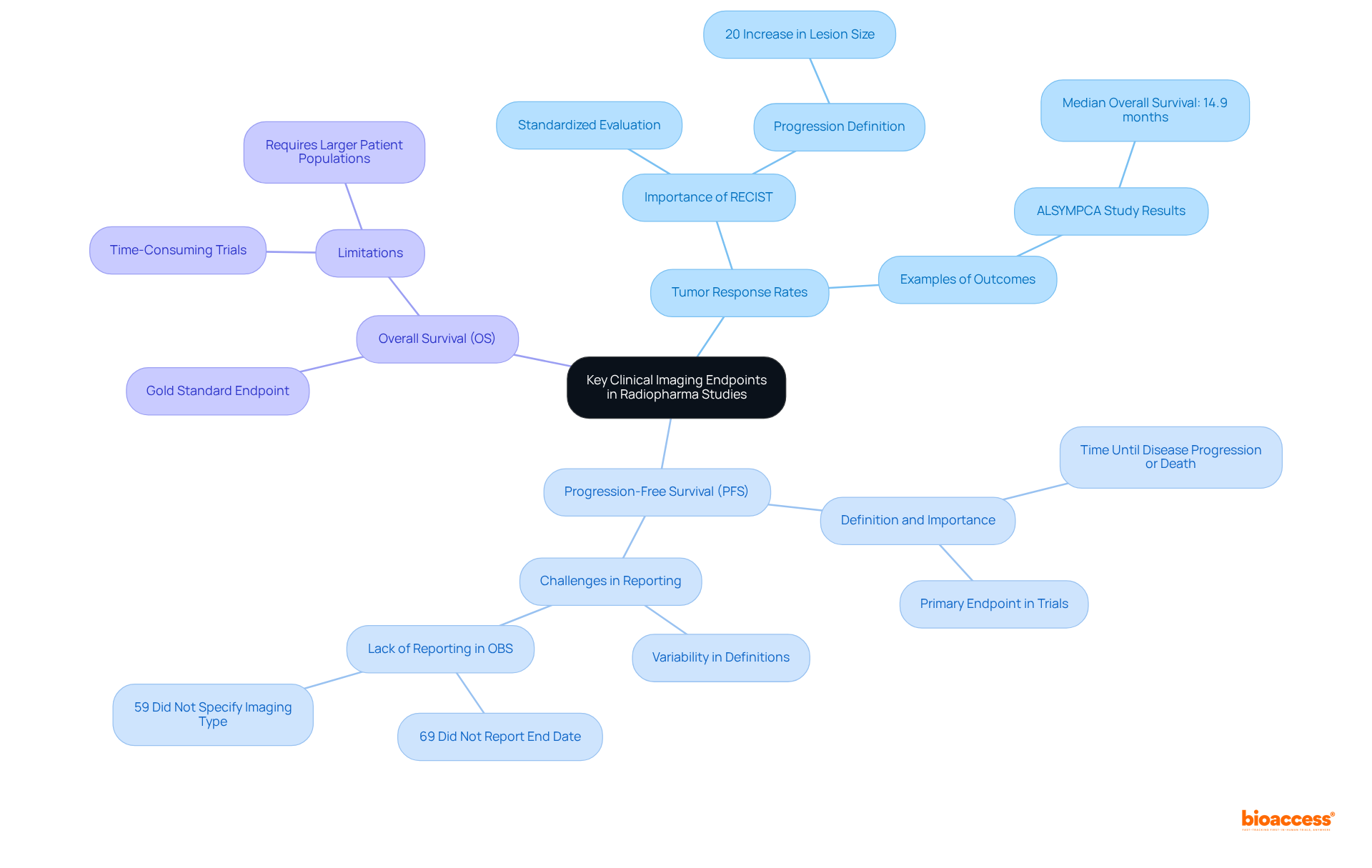
To ensure compliance with regulatory and ethical standards, researchers must possess a comprehensive understanding of the guidelines established by organizations such as the FDA and EMA. This encompasses specific requirements for radiopharmaceuticals, including critical aspects like labeling, storage, and disposal. The paramount importance of obtaining informed consent from participants cannot be overstated, particularly in light of the inherent risks associated with radiation exposure. Statistics indicate that informed consent rates in studies involving radiation can vary significantly, underscoring the necessity for clear communication regarding potential risks and benefits.
Furthermore, regular audits and training sessions for the research team are essential to uphold compliance and proactively address any ethical concerns. By leveraging thorough research study management services—ranging from feasibility assessments and site selection to compliance evaluations, study setup, import permits, project management, and reporting on study status, including serious and non-serious adverse events—researchers can significantly enhance the credibility of their investigations.
Prioritizing adherence to these standards not only safeguards participant welfare but also cultivates trust in the clinical research process.
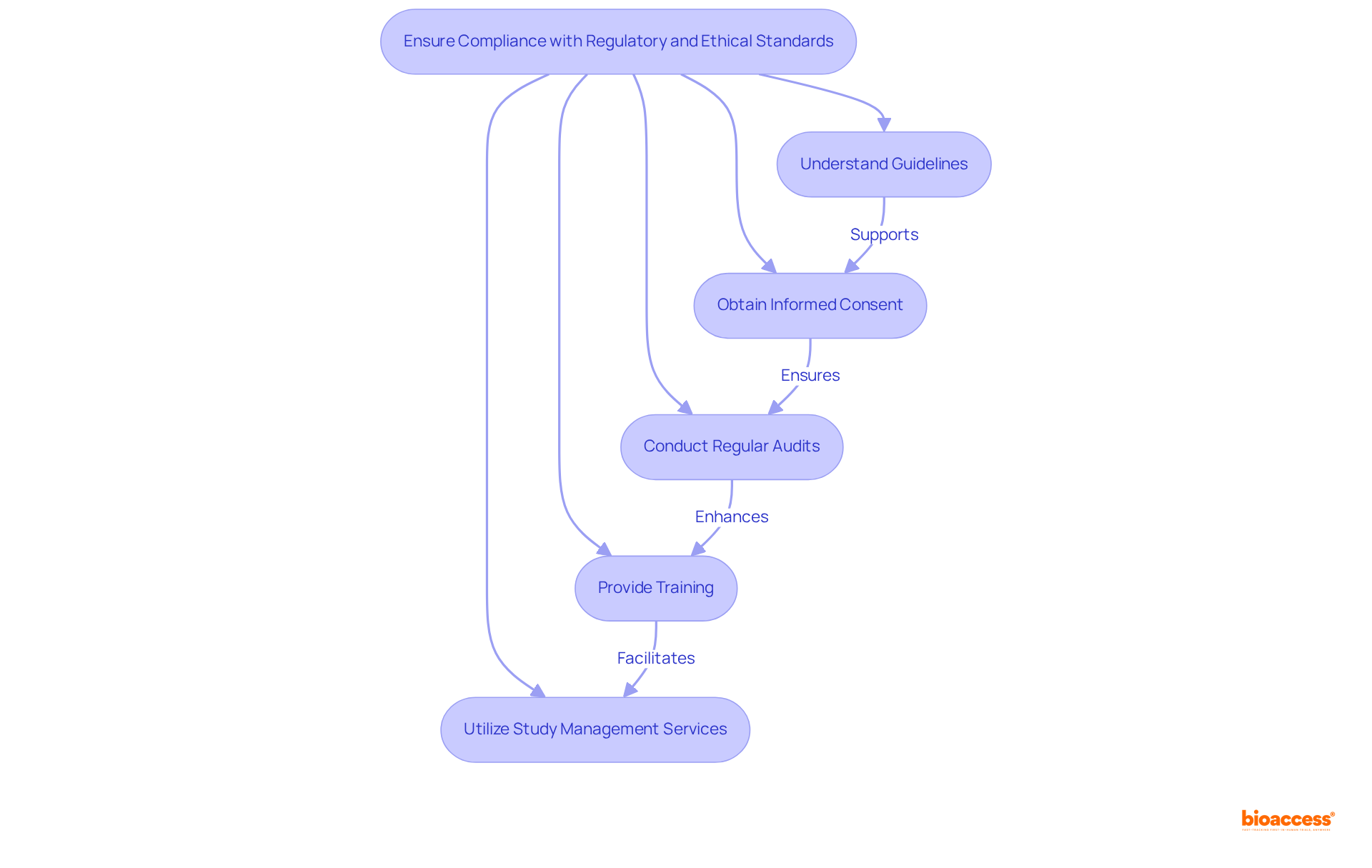
To enhance patient recruitment and retention in studies focused on radiopharma clinical imaging endpoints, a multifaceted approach is essential. This strategy should leverage digital platforms for outreach, tapping into their ability to reach a broader audience and engage potential participants effectively. Collaborating with patient advocacy organizations can further improve outreach initiatives, ensuring that details about the study's advantages and hazards are conveyed clearly and accessibly.
Maintaining open lines of communication with participants throughout the research is vital for fostering trust and promoting continual involvement. Regular updates and check-ins can help participants feel valued and informed. Moreover, introducing adaptable scheduling choices and reducing the strain of participation can significantly enhance retention rates. For instance, providing transportation assistance or offering telehealth options for follow-up visits can boost participant satisfaction and commitment to the study.
Effective retention tactics in radiopharmaceutical studies also involve addressing common obstacles to involvement in radiopharma clinical imaging endpoints. By recognizing the distinct difficulties encountered by patients, such as lack of awareness or concerns regarding the process, researchers can tailor their methods to meet these needs. Involving clinical research coordinators in discussions about enhancing patient retention can yield valuable insights, as they often possess firsthand experience with participant concerns and motivations. Ultimately, a patient-centric approach that prioritizes convenience and support can lead to more successful recruitment and retention outcomes for radiopharma clinical imaging endpoints in radiopharmaceutical trials.
Mastering the intricacies of radiopharmaceutical clinical imaging endpoints is essential for advancing medical research and improving patient outcomes. A comprehensive understanding of radiopharmaceuticals, their roles in diagnostics and therapy, and the significance of precise imaging endpoints lays the foundation for successful clinical trials. By harnessing the power of these specialized agents, healthcare professionals can achieve more accurate diagnoses and tailor treatments to meet individual patient needs.
Key insights from the article highlight the importance of identifying relevant clinical imaging endpoints, such as:
These metrics, coupled with adherence to regulatory and ethical standards, ensure that studies yield reliable and applicable results. Furthermore, effective patient recruitment and retention strategies are crucial in overcoming the challenges faced in clinical trials, ultimately fostering a robust research environment that prioritizes participant welfare and engagement.
As the field of radiopharmaceuticals continues to evolve, the importance of mastering clinical imaging endpoints cannot be overstated. Researchers and healthcare professionals are encouraged to embrace innovative strategies and maintain high standards of compliance. By doing so, they can contribute to the advancement of personalized medicine and improve the overall effectiveness of radiopharmaceutical therapies, paving the way for a brighter future in patient care and treatment outcomes.
What are radiopharmaceuticals?
Radiopharmaceuticals are specialized agents that incorporate radioactive isotopes and serve both diagnostic and therapeutic functions in medicine.
What is the primary role of radiopharmaceuticals in medical imaging?
Their primary role is to provide intricate insights into physiological processes, enabling healthcare professionals to diagnose diseases with precision.
Which radiopharmaceutical is considered a cornerstone in imaging modalities?
Technetium-99m is considered a cornerstone in imaging modalities such as SPECT (Single Photon Emission Computed Tomography).
What is the significance of Technetium-99m in SPECT imaging?
Technetium-99m is utilized to assess organ function and identify abnormalities, and it is projected to dominate SPECT imaging in 2025.
Why is understanding pharmacokinetics and biodistribution important for radiopharmaceuticals?
Understanding pharmacokinetics and biodistribution is crucial for designing effective clinical trials and accurately interpreting radiopharma clinical imaging endpoints.
How do radiopharmaceuticals contribute to personalized medicine?
Knowledge of radiopharmaceuticals enhances the effectiveness of radioactive drugs and propels the advancement of personalized medicine, allowing for targeted therapies customized to individual patient needs.
What is the projected market growth for radioactive pharmaceuticals?
The market for radioactive pharmaceuticals is anticipated to expand at a compound annual growth rate (CAGR) of 16.4% from 2024 to 2029, with forecasts suggesting a market size of USD 19.6 billion by 2034.
In which medical fields are radiopharmaceuticals particularly impactful?
Radiopharmaceuticals offer highly targeted diagnostic and therapeutic solutions, particularly in oncology, cardiology, and neurology.
What challenges do radiopharmaceuticals face in the field?
Significant challenges include the short half-lives of isotopes and high production costs.