


This article serves as a comprehensive guide for Clinical Research Directors aiming to master the Recommended Phase 2 Dose (RP2D). It outlines essential steps to define the RP2D, including:
Each step is underpinned by critical considerations such as:
This ensures that the RP2D is both effective and safe for patient administration in clinical trials.
Defining the Recommended Phase 2 Dose (RP2D) stands as a pivotal step in the clinical research landscape, where precision significantly influences patient safety and trial outcomes. This guide explores the essential processes that clinical research directors must navigate, from gathering preclinical data to designing robust trial protocols. Yet, a critical challenge persists: how can researchers ensure that their chosen RP2D strikes a balance between efficacy and tolerability while adhering to stringent regulatory standards? This article offers a comprehensive roadmap, equipping directors with the insights necessary to master the complexities of RP2D determination and enhance the success of their clinical trials.
To define the rp2d, it is essential to start with a comprehensive review of the drug's pharmacokinetics and pharmacodynamics. This involves consulting existing literature and prior clinical study data to identify the maximum tolerated dose (MTD) established during Phase 1 evaluations.
Collaboration with pharmacologists is crucial to gain a comprehensive understanding of the drug's mechanism of action and its therapeutic window. The dose represented by rp2d must be both effective and tolerable, ensuring safe administration to patients in Phase 2 trials.
It is imperative to document the rationale behind the chosen rp2d, as this documentation will be critical for regulatory submissions and ethical approvals.
To effectively gather preclinical data, it is essential to compile all relevant studies, including both in vitro and in vivo research. Focus on research that distinctly demonstrates the drug's effectiveness, risk profile, and any noted side effects. For instance, utilizing 500 ng of RNA for library preparation can provide a robust foundation for understanding the drug's molecular interactions. Collaborate closely with toxicologists to assess potential risks linked to the drug, ensuring a comprehensive evaluation of its safety. As highlighted by Diego Galeano, "The interactions between miRNAs and viruses have revealed a multifaceted relationship," underscoring the importance of toxicological insights in drug evaluation. Carefully examine the information to unveil dose-response relationships and identify any adverse effects that may influence clinical study design. For example, the IC50 of the anti-miR-2392 nanoligomer is 1.15 ± 0.33 μM, illustrating the significance of thorough preclinical assessments. This comprehensive understanding is vital for making informed decisions that prioritize patient safety and enhance study outcomes. Furthermore, consider utilizing tools such as the KAPA Hyper Library Preparation Kit for efficient library preparation and data analysis.
Creating a research study protocol requires a comprehensive outline of the study's goals, structure, methods, and statistical analysis plan. Essential elements include:
It is imperative to ensure that the protocol adheres to all regulatory requirements and ethical standards. Collaborating with biostatisticians is essential for determining the appropriate sample size and statistical methods, which are critical for maintaining the integrity of the study. Engaging with key stakeholders, including regulatory bodies and ethics committees, during the review process guarantees that the protocol is clear, compliant, and prepared for implementation. Recent advancements in research methodology underscore the necessity of robust statistical analysis plans, which are fundamental for achieving reliable results and justifying sample size determinations. By adhering to these guidelines, researchers can enhance the scientific validity of their studies and improve the likelihood of successful outcomes.

Defining clear inclusion and exclusion criteria is crucial for the success of medical studies, particularly regarding the drug's mechanism of action and the specific disease being targeted. Factors such as age, gender, disease stage, and previous treatment history must be carefully considered to ensure that the selected patient population is appropriate.
Collaborating with clinical teams throughout this process is essential; their insights can aid in refining these criteria for practical application in the study context. This collaborative method not only helps in recruiting the appropriate patients but also improves the significance and dependability of research outcomes.
Statistics indicate that well-defined criteria can greatly enhance recruitment efficiency, as studies with clear guidelines are more likely to achieve their enrollment targets. Furthermore, addressing the complexities of inclusion and exclusion criteria can reduce the risk of underpowered studies, which frequently occur due to excessive dropouts and can lead to ethical concerns regarding study validity.
By prioritizing these criteria, clinical research directors can enhance study design and execution, ultimately contributing to more successful outcomes.
When applying dose escalation approaches, selecting a suitable design—such as the 3+3 design or accelerated titration—that aligns with the study's goals and the drug's risk profile is essential. To ensure safety and efficacy, it is imperative to closely monitor patient responses and adjust doses as necessary. This process must be supported by extensive research study management services, including:
These services play a critical role in identifying the most appropriate research locations and primary investigators. Furthermore, acquiring import permits and ensuring adherence to the ethics committee during study preparation are vital for upholding regulatory standards. Effective communication with the medical team is crucial to swiftly address any adverse occurrences, and comprehensive documentation of all dosage modifications and the rationale behind them will be indispensable for analysis and regulatory reporting.
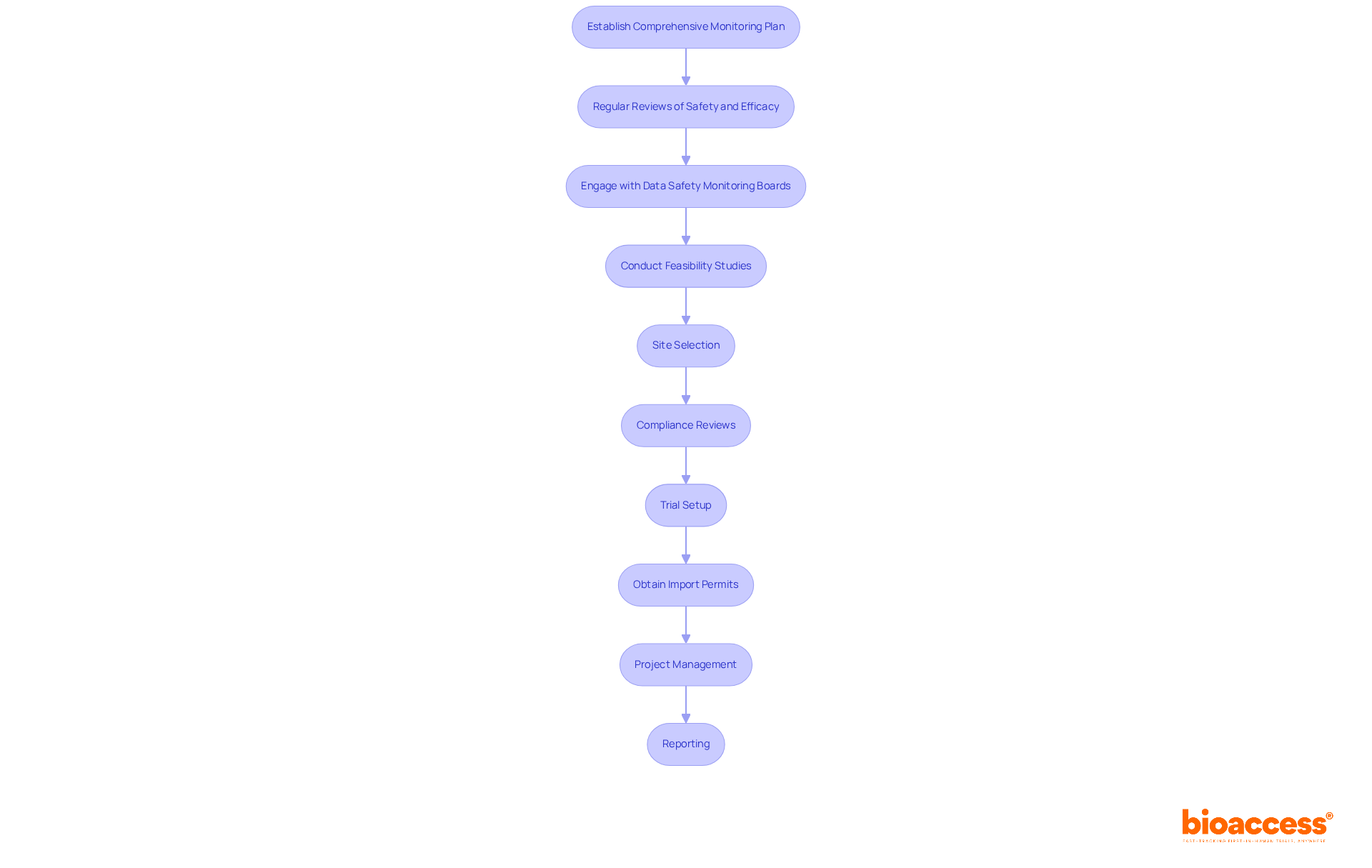
To ensure the integrity and safety of clinical studies, establishing a comprehensive monitoring plan is essential. This plan must include regular reviews of both safety and efficacy information. According to the ICH E9 guidelines, clarity in study objectives and consideration of intercurrent events are crucial for accurately describing treatment effects. Furthermore, utilizing advanced statistical software for real-time data analysis enables the rapid identification of trends or potential issues, facilitating timely interventions.
Engaging with Data Safety Monitoring Boards (DSMB) is vital for maintaining independent oversight of study safety. These boards provide expert guidance and ensure that participant welfare is prioritized throughout the research. For instance, the Data Monitoring Committee (DMC) for the RECOVERY study has convened as frequently as every two weeks to ensure ongoing oversight. Additionally, meticulous documentation of all findings and decisions derived from data analysis is critical, as this information will be essential for final reporting and regulatory submissions.
Moreover, extensive trial management services—such as:
play a crucial role in enhancing the reliability of trials. By implementing these strategies, clinical research directors can ensure compliance with regulatory standards while improving the overall effectiveness of their clinical studies.
Defining the Recommended Phase 2 Dose (RP2D) is a pivotal step in the clinical research process, ensuring that drug administration is both effective and safe for patients. This guide underscores the necessity of a systematic approach, commencing with thorough literature reviews and preclinical data analysis, leading to the meticulous design of clinical trial protocols. By adhering to these steps, clinical research directors can establish a robust foundation for successful Phase 2 trials.
Key insights discussed highlight the importance of collaboration with various experts, including pharmacologists and biostatisticians, to gather comprehensive data and create robust study designs. Establishing clear patient selection criteria and implementing effective dose escalation strategies further enhance the integrity of clinical trials. Continuous monitoring and analysis of trial data are essential for maintaining participant safety and ensuring regulatory compliance.
Ultimately, mastering the RP2D process is vital for advancing drug development and improving patient outcomes. By following the best practices outlined in this guide, clinical research directors can confidently navigate the complexities of clinical trials, contributing to the overall success of therapeutic innovations. The commitment to meticulous planning and execution not only fosters scientific validity but also reinforces the ethical responsibility to prioritize patient safety throughout the research journey.
What is the Recommended Phase 2 Dose (RP2D)?
The RP2D is the dose of a drug determined to be both effective and tolerable for administration to patients in Phase 2 clinical trials, based on a comprehensive review of the drug's pharmacokinetics and pharmacodynamics.
How is the RP2D defined?
The RP2D is defined by reviewing existing literature and prior clinical study data to identify the maximum tolerated dose (MTD) established during Phase 1 evaluations, and by collaborating with pharmacologists to understand the drug's mechanism of action and therapeutic window.
Why is documenting the rationale behind the RP2D important?
Documenting the rationale for the chosen RP2D is critical for regulatory submissions and obtaining ethical approvals for clinical trials.
What types of preclinical data are important for drug evaluation?
Important preclinical data includes both in vitro and in vivo studies that demonstrate the drug's effectiveness, risk profile, and noted side effects, as well as dose-response relationships and potential adverse effects.
How can researchers gather preclinical data effectively?
Researchers can gather preclinical data by compiling relevant studies, collaborating with toxicologists to assess safety risks, and utilizing tools like the KAPA Hyper Library Preparation Kit for efficient library preparation and data analysis.
What role do toxicologists play in drug evaluation?
Toxicologists collaborate with researchers to assess potential risks linked to the drug, ensuring a comprehensive evaluation of its safety and helping to inform clinical study design.
Can you provide an example of a preclinical assessment finding?
An example of a preclinical assessment finding is the IC50 of the anti-miR-2392 nanoligomer, which is 1.15 ± 0.33 μM, illustrating the importance of thorough preclinical evaluations in understanding drug interactions and effects.