


The article underscores the pivotal role of an integrated data platform in amplifying clinical research success. By streamlining data management, enhancing decision-making, and ensuring regulatory compliance, these platforms fundamentally transform research processes. They provide real-time access to unified datasets, which not only reduces operational costs but also fosters improved patient engagement. This comprehensive approach ultimately leads to more efficient and effective research outcomes, positioning integrated data platforms as essential tools in the modern Medtech landscape.
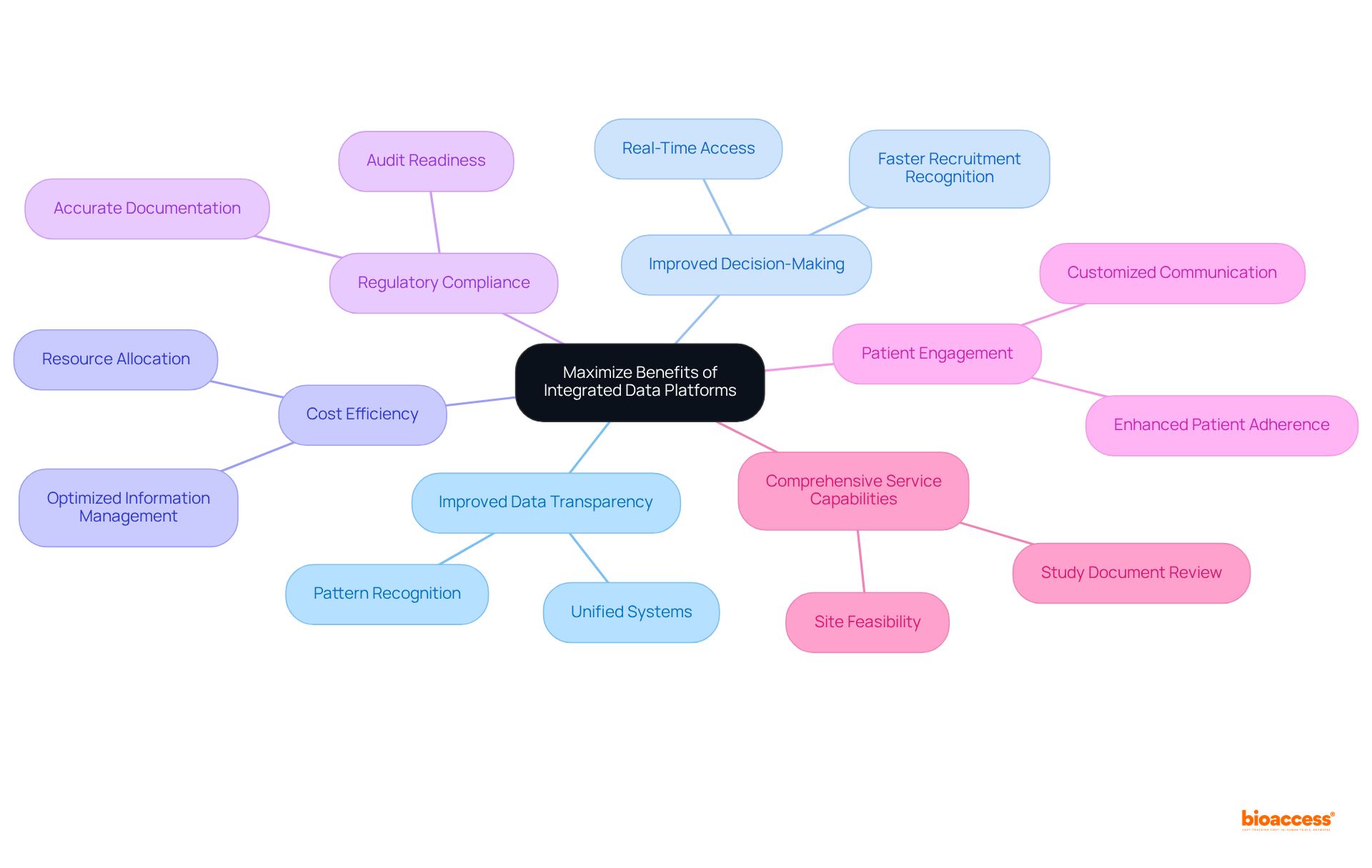
Integrated data platforms are revolutionizing the landscape of clinical research, providing a unified approach to managing diverse data sources that significantly enhance both efficiency and accuracy. By consolidating information from clinical study management systems, electronic data capture systems, and laboratory information management systems, these platforms empower researchers to navigate complex trials with increased ease and reliability.
As the industry transitions towards more patient-centric and decentralized models, organizations must consider how to effectively leverage these integrated systems. This not only streamlines operations but also drives innovation and ensures compliance in their research efforts.
Integrated information systems in clinical research serve as comprehensive frameworks that consolidate data from various sources, including clinical study management systems (CTMS), electronic data capture (EDC) systems, and laboratory information management systems (LIMS). These systems facilitate immediate access to information, empowering researchers to effectively track trial progress, manage patient data, and assess results. By merging diverse information sources, an integrated data platform significantly reduces the risk of errors, enhances data integrity, and streamlines reporting processes. They also play a crucial role in ensuring regulatory compliance by maintaining accurate and auditable records, which are essential for successful study outcomes and building stakeholder confidence.
The importance of these systems is underscored by industry experts, with one noting that a purpose-built life sciences information management solution can employ a multi-domain approach to manage enterprise information for consistent, reliable, and faster access to insights. Furthermore, McKinsey estimates that predictive analytics could reduce testing costs by 15-25%, highlighting the financial advantages of adopting an integrated data platform.
Successful implementations of an integrated data platform have demonstrated their capacity to enhance trial efficiency. For example, organizations utilizing such systems have reported notable improvements in patient care outcomes, illustrating the vital role of an integrated data platform in achieving healthcare objectives. As the landscape evolves, the integration of an integrated data platform with artificial intelligence (AI) and machine learning (ML) into these systems is expected to further optimize information management, increase precision, and reduce labor-intensive tasks, ultimately enhancing efficiency in healthcare information management.
By 2025, the focus on unified information systems will be paramount, as over 70% of sponsors and CROs plan to incorporate digital and decentralized elements in forthcoming studies. This shift towards patient-centric frameworks utilizing real-world evidence (RWE) will render research studies more adaptable to changing needs, ensuring that integrated data platforms remain at the forefront of research management. To maximize the benefits of these advancements, consider partnering with bioaccess® for comprehensive trial management services, including feasibility studies, site selection, compliance reviews, trial setup, import permits, project management, and reporting.
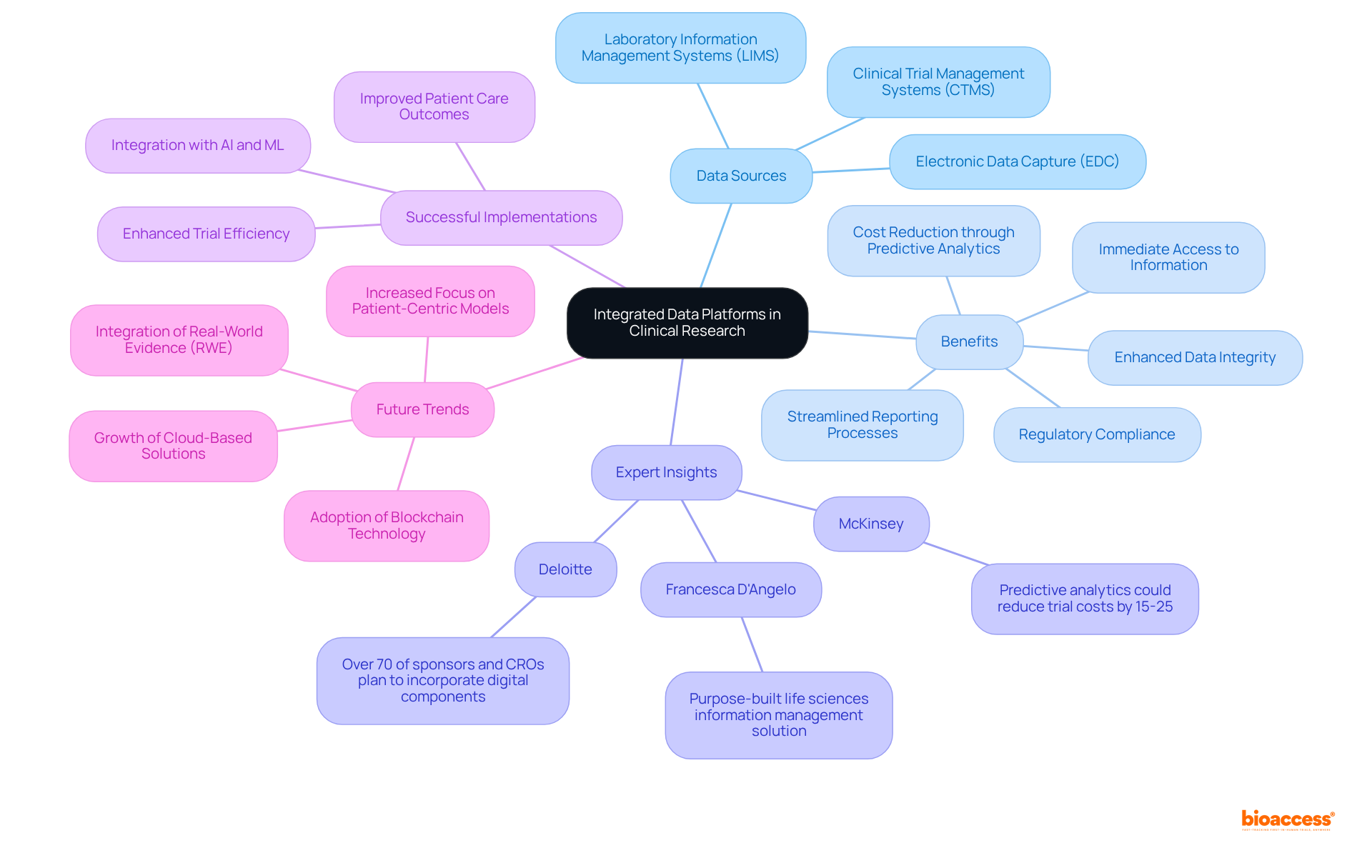
To implement best practices for information integration in clinical research, it is crucial to adopt effective strategies that leverage an integrated data platform.
Standardize Information Formats: Establishing uniform information formats across all systems facilitates seamless transfer and minimizes discrepancies. Standardization significantly improves information quality, thereby enhancing the integration and analysis of details from various sources within an integrated data platform.
Utilize APIs: Leveraging application programming interfaces (APIs) automates information exchange between systems, reducing manual entry and the potential for mistakes. This automation streamlines workflows and enhances information accuracy.
Regular Information Audits: Conducting periodic assessments ensures accuracy and integrity, allowing for the prompt identification and correction of discrepancies. Regular audits are essential for maintaining high standards of information quality, which is critical for reliable research outcomes. Training and support through extensive instruction for personnel on effectively utilizing an integrated data platform ensures they comprehend the significance of information quality and compliance. Well-trained personnel are vital for maximizing the benefits of integrated systems.
Collaborative Tools: Implementing collaborative tools that enable real-time information sharing among team members enhances communication and decision-making. These tools promote a culture of openness and teamwork, which is vital for successful trials.
By following these practices, organizations can significantly improve their information integration efforts, leading to more reliable and impactful research outcomes.
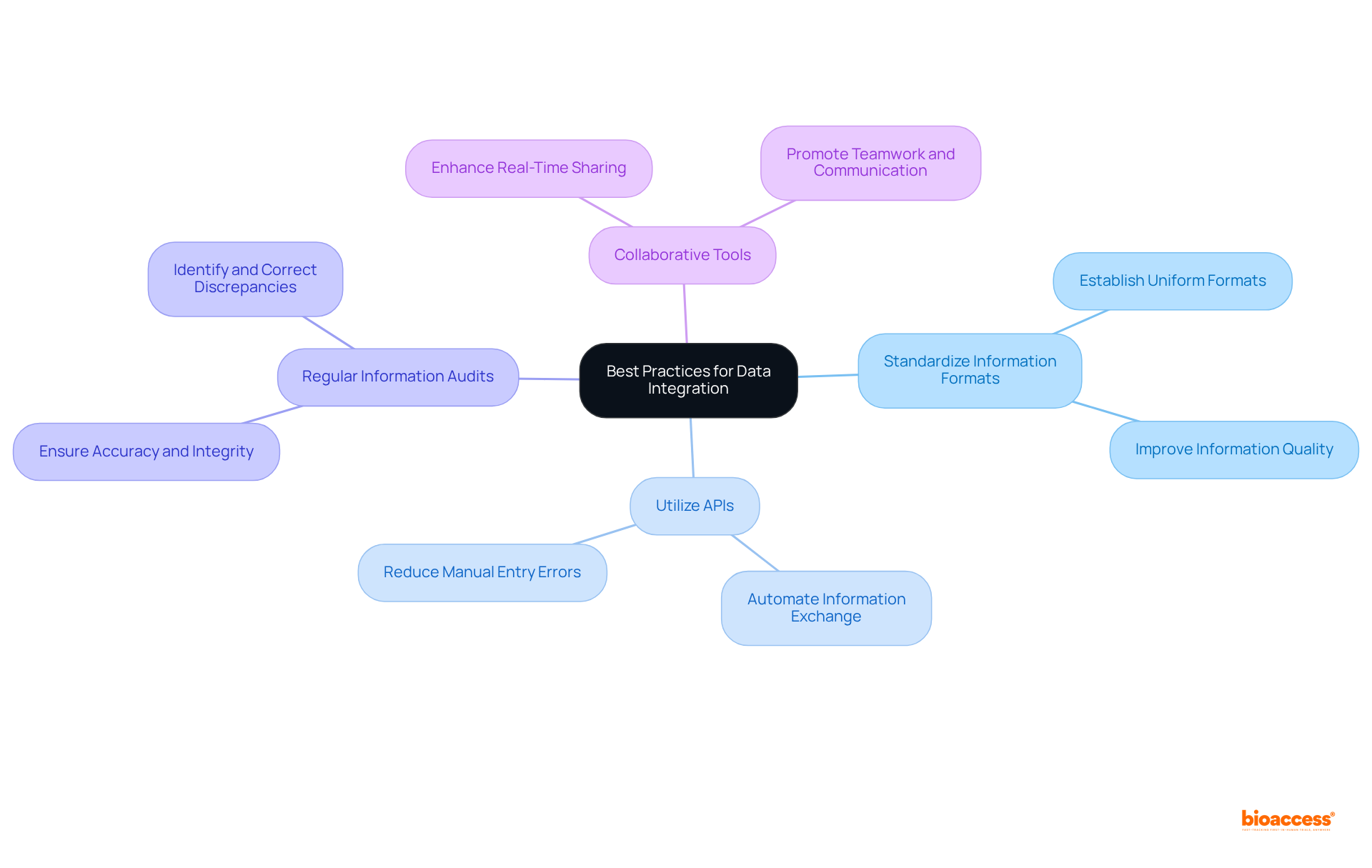
To fully leverage the advantages of integrated data platforms in clinical research, organizations should prioritize the following key areas:
Improved Data Transparency: Unified systems provide a thorough perspective of all information, enabling researchers to quickly recognize patterns and extract practical insights. This visibility is crucial for timely decision-making, especially in fast-paced clinical environments.
Improved Decision-Making: With real-time access to a unified dataset, stakeholders can make informed decisions more rapidly, significantly reducing the time required to bring new therapies to market. For example, systems such as Trial360 have shown that improved information visibility results in faster recognition of recruitment setbacks and protocol adherence problems, promoting better overall trial results.
Cost Efficiency: Optimizing information management procedures through unified systems can result in significant decreases in operational expenses related to information handling and analysis. By automating routine tasks and centralizing information, organizations can allocate resources more effectively, enhancing productivity.
Regulatory Compliance: Integrated platforms play a vital role in maintaining compliance with regulatory standards. They guarantee that all information is accurately documented and easily accessible for audits, which is crucial in an industry where the typical expense of a security breach can approach nearly $11 million. This compliance is particularly critical in regions like Colombia, where adherence to INVIMA regulations is necessary for medical device oversight and classification.
Patient Engagement: Improved information management capabilities allow researchers to create more effective patient engagement strategies. By examining patient information, organizations can customize communication and support, ultimately enhancing patient adherence and satisfaction.
Comprehensive Service Capabilities: Organizations should also focus on the feasibility and selection of research sites and principal investigators (PIs), as well as the review and feedback on study documents to ensure compliance with country requirements. Effective trial setup, start-up, and approval processes, including obtaining import permits and nationalizing investigational devices, are crucial for successful project management and monitoring.
By concentrating on these areas, organizations can maximize the potential of an integrated data platform, driving success in their clinical research initiatives and fostering innovation in the Medtech, Biopharma, and Radiopharma sectors.
The integration of data platforms in clinical research represents a transformative force, streamlining processes and enhancing the quality of study outcomes. By consolidating diverse data sources into a unified system, researchers gain immediate access to vital information that not only improves data integrity but also fosters regulatory compliance and stakeholder confidence. An integrated data platform is not merely a technological upgrade; it is a strategic necessity for organizations aiming to navigate the complexities of modern clinical trials effectively.
Key insights from the article highlight the multifaceted benefits of adopting integrated data platforms. These systems facilitate improved data transparency, enabling rapid decision-making and ultimately reducing the time to market for new therapies. Furthermore, the cost efficiency gained through automation and centralized information management is significant, allowing organizations to allocate resources more effectively. As the industry shifts towards patient-centric frameworks, the role of integrated data platforms becomes even more critical in ensuring successful outcomes and enhancing patient engagement.
In light of these findings, it is imperative for organizations to embrace the best practices for data integration outlined in the article. By standardizing information formats, utilizing APIs, conducting regular audits, and fostering collaboration, research teams can unlock the full potential of integrated data platforms. As the landscape of clinical research evolves, those who prioritize these strategies will not only enhance their operational efficiency but also contribute to the advancement of healthcare innovation. Embracing integrated data platforms is not just a choice; it is a pathway to maximizing success in clinical research.
What are integrated data platforms in clinical research?
Integrated data platforms are comprehensive frameworks that consolidate data from various sources, such as clinical study management systems (CTMS), electronic data capture (EDC) systems, and laboratory information management systems (LIMS), to provide immediate access to information for researchers.
How do integrated data platforms benefit clinical research?
They empower researchers to track trial progress, manage patient data, assess results, reduce the risk of errors, enhance data integrity, streamline reporting processes, and ensure regulatory compliance by maintaining accurate and auditable records.
What financial advantages do integrated data platforms offer?
According to McKinsey, predictive analytics enabled by integrated data platforms could reduce testing costs by 15-25%.
What improvements have been reported from using integrated data platforms?
Organizations utilizing integrated data platforms have reported notable improvements in trial efficiency and patient care outcomes, demonstrating their vital role in achieving healthcare objectives.
How is artificial intelligence (AI) and machine learning (ML) expected to impact integrated data platforms?
The integration of AI and ML is expected to optimize information management, increase precision, and reduce labor-intensive tasks, ultimately enhancing efficiency in healthcare information management.
What is the future focus for sponsors and CROs regarding integrated data platforms?
By 2025, over 70% of sponsors and CROs plan to incorporate digital and decentralized elements in their studies, focusing on unified information systems and patient-centric frameworks that utilize real-world evidence (RWE).
How can organizations maximize the benefits of integrated data platforms?
Organizations can maximize the benefits by partnering with bioaccess® for comprehensive trial management services, which include feasibility studies, site selection, compliance reviews, trial setup, import permits, project management, and reporting.