


This article presents essential insights for navigating the European Medicines Agency (EMA) regulatory landscape in clinical research. It underscores the EMA's critical role in ensuring the safety and effectiveness of medicines through a centralized authorization process, rigorous data requirements, and ethical considerations. Understanding these regulations is paramount for the success of clinical trials, as it lays the groundwork for compliance and operational excellence in the Medtech landscape.
The European Medicines Agency (EMA) serves as a cornerstone in the realm of pharmaceutical regulation, ensuring that medicines across the EU adhere to stringent safety and efficacy standards. As clinical researchers navigate this intricate regulatory landscape, grasping the EMA's framework and processes is essential for the successful execution and approval of studies.
However, with evolving guidelines and heightened scrutiny from the EMA, how can researchers effectively adapt their strategies to not only comply but also excel in this demanding environment? This article explores essential insights and practical approaches for leveraging EMA regulations to enhance clinical research outcomes.
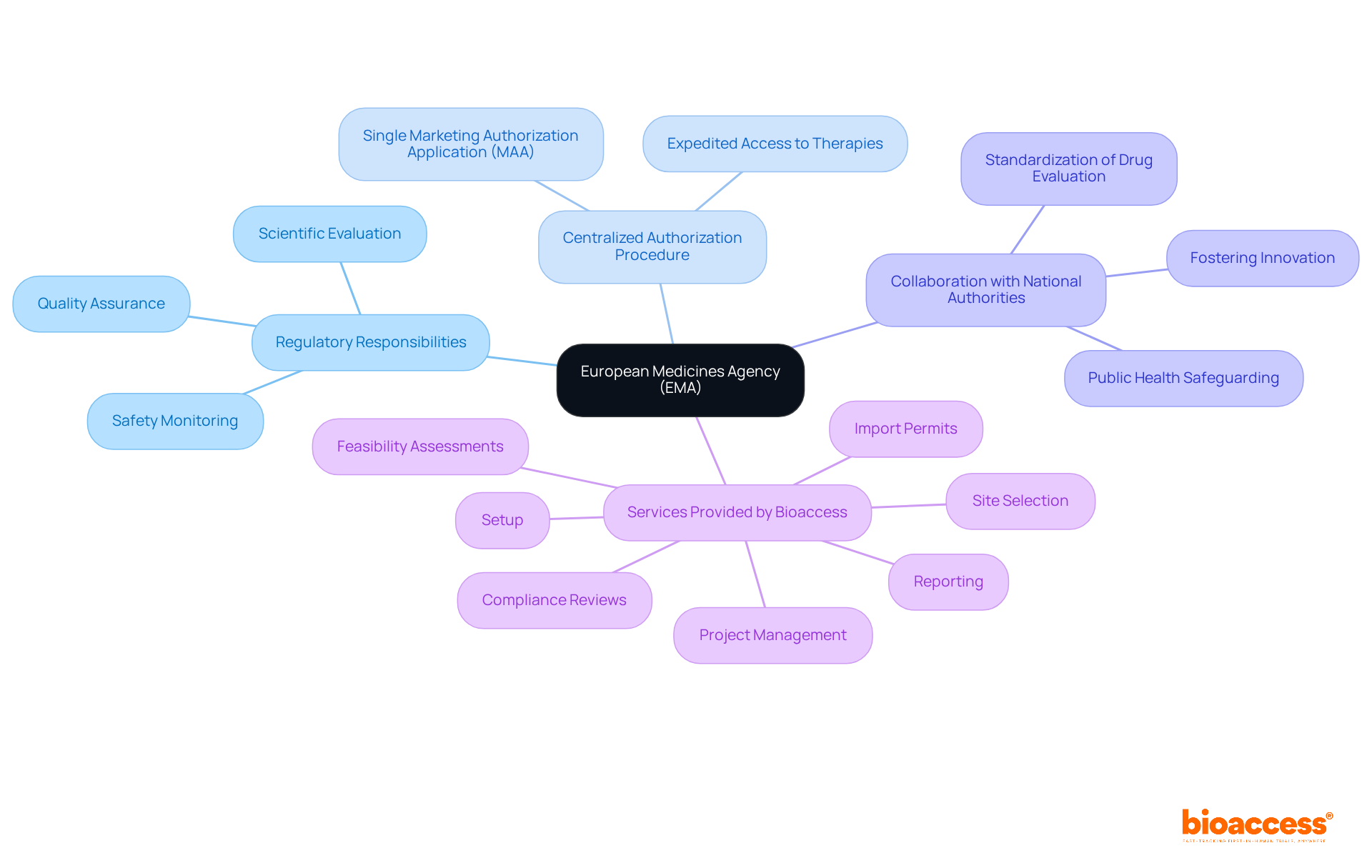
The ema regulatory agency serves as a pivotal authority within the European Union, tasked with the scientific evaluation, supervision, and safety monitoring of medicines. Since its establishment in 1995, the ema regulatory agency has been dedicated to ensuring that all medicines available in the EU are safe, effective, and of the highest quality. The agency is instrumental in the centralized authorization procedure, which enables pharmaceutical companies to submit a single marketing authorization application (MAA) for access to the entire EU market. This streamlined process is essential for expediting the availability of new therapies to patients throughout Europe.
Moreover, the ema regulatory agency collaborates closely with national regulatory authorities to standardize the processes of drug evaluation and approval. This collaboration not only safeguards public health but also fosters innovation within the pharmaceutical sector. In this context, bioaccess provides comprehensive management services for studies, including:
With experts like Katherine Ruiz, who specializes in regulatory affairs for medical devices and in vitro diagnostics in Colombia, bioaccess ensures that studies adhere to the EMA regulatory agency requirements. This compliance facilitates smoother approvals and contributes positively to local economies through job creation, economic growth, and enhanced healthcare.
The EMA regulatory agency operates under a comprehensive regulatory framework that governs the approval of medicinal products through various directives and regulations. Understanding this framework is essential for researchers and sponsors navigating the complex regulatory environment.
Key components of this framework include:
Grasping these procedures is vital for efficiently maneuvering through the regulatory landscape and ensuring adherence to EMA regulatory agency standards.

The EMA regulatory agency regulations profoundly influence medical research, shaping study design, execution, and reporting. Their implications are significant and multifaceted:
Heightened Inspection: The EMA's rigorous assessment procedure mandates that medical studies are meticulously structured and executed to meet enhanced safety and effectiveness criteria. This increased scrutiny from the EMA regulatory agency has led to a notable rise in the number of assessments requiring additional documentation and justification for their methodologies.
Data Requirements: Researchers must compile extensive data packages, encompassing both preclinical and trial data. This comprehensive approach can be labor-intensive, often extending timelines significantly. For instance, the average expense of a Phase II clinical study can range from $5 million to $15 million, reflecting the extensive data preparation needed to satisfy the EMA regulatory agency requirements.
Patient Safety and Ethical Considerations: The EMA prioritizes the safeguarding of study participants, necessitating robust ethical supervision and informed consent measures. This emphasis on patient safety has resulted in more stringent guidelines regarding participant recruitment and oversight, ensuring that ethical standards are upheld throughout the study.
Timelines and costs can be prolonged and escalated when navigating the procedures of the EMA regulatory agency. Sponsors must strategically plan and allocate resources to accommodate these potential delays. The financial implications are considerable, as research studies in the U.S. are among the most costly globally, with expenses influenced by regulatory adherence and operational requirements.
Adaptation to regulatory changes is crucial, as researchers must remain vigilant and adjust their practices in response to the evolving standards set by the EMA regulatory agency. This includes understanding the implications of the Clinical Trials Regulation and the transition to the Clinical Trials Information System (CTIS), which aims to streamline processes but necessitates a thorough familiarity with new protocols.
In this context, Bioaccess provides extensive management services for studies, including feasibility assessments, site selection, compliance reviews, setup, import permits, project oversight, and reporting. Katherine Ruiz, an expert in regulatory affairs for medical devices and in vitro diagnostics in Colombia, plays a crucial role in guiding researchers through these complexities. By acknowledging these implications and leveraging the expertise of professionals like Ruiz, clinical researchers can navigate the intricacies of EMA regulations more effectively, ultimately enhancing the likelihood of successful trial outcomes.

The European Medicines Agency (EMA) is pivotal in ensuring that medicines available within the EU are safe, effective, and of high quality. By streamlining the marketing authorization process and collaborating with national authorities, the EMA enhances public health and fosters innovation in the pharmaceutical sector. For researchers and sponsors, understanding the EMA's regulatory framework is essential, as it shapes the design, execution, and reporting of clinical studies.
Key insights from this exploration reveal the rigorous assessment procedures, extensive data requirements, and ethical considerations mandated by the EMA. These factors significantly influence the timelines and costs associated with clinical research, necessitating strategic planning and resource allocation. Moreover, the importance of adapting to evolving regulations, such as the Clinical Trials Regulation and the transition to the Clinical Trials Information System (CTIS), cannot be overstated. Engaging with experts in regulatory affairs, such as those at Bioaccess, can facilitate compliance and enhance the likelihood of successful trial outcomes.
In conclusion, navigating the complexities of EMA regulations is crucial for clinical researchers aiming to contribute to medical advancements. By prioritizing regulatory adherence and leveraging expert guidance, researchers can meet the stringent demands of the EMA while driving innovation that ultimately benefits public health. The significance of understanding and complying with EMA guidelines is foundational for successful clinical research and the development of new therapies that improve patient outcomes across Europe.
What is the role of the European Medicines Agency (EMA)?
The EMA serves as a pivotal authority within the European Union, responsible for the scientific evaluation, supervision, and safety monitoring of medicines.
When was the EMA established?
The EMA was established in 1995.
What is the purpose of the centralized authorization procedure?
The centralized authorization procedure allows pharmaceutical companies to submit a single marketing authorization application (MAA) for access to the entire EU market, expediting the availability of new therapies to patients.
How does the EMA collaborate with national regulatory authorities?
The EMA collaborates closely with national regulatory authorities to standardize the processes of drug evaluation and approval, which helps safeguard public health and foster innovation in the pharmaceutical sector.
What services does Bioaccess provide in relation to EMA requirements?
Bioaccess provides comprehensive management services for studies, including feasibility assessments, site selection, compliance reviews, setup, import permits, project management, and reporting.
Who is Katherine Ruiz and what is her expertise?
Katherine Ruiz is an expert specializing in regulatory affairs for medical devices and in vitro diagnostics in Colombia, ensuring that studies comply with EMA requirements.
How does compliance with EMA regulations benefit local economies?
Compliance with EMA regulations facilitates smoother approvals, which contributes positively to local economies through job creation, economic growth, and enhanced healthcare.