


Navigating the complex landscape of biological product regulation in Australia presents significant challenges for researchers and organizations. The Therapeutic Goods Administration (TGA) has laid out comprehensive guidelines essential for ensuring the safety and efficacy of biologicals. However, many are left questioning how to effectively comply with these regulations.
This guide provides a step-by-step approach to mastering TGA's Biologicals Guidelines, equipping readers with the knowledge needed to streamline their research processes and enhance compliance.
What common pitfalls can derail even the most prepared teams, and how can they be avoided?
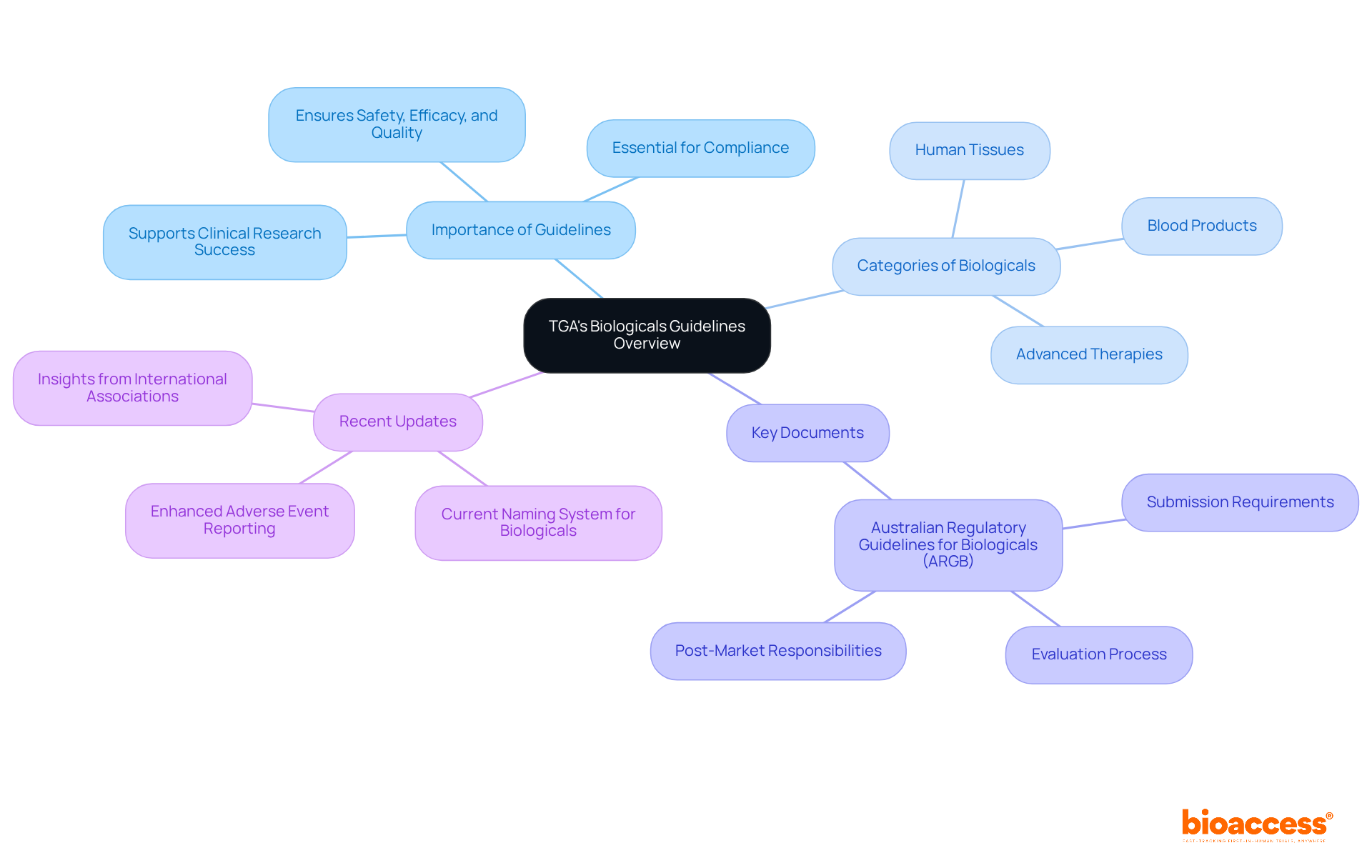
The Therapeutic Goods Administration (TGA) plays a crucial role in regulating biologicals in Australia, ensuring the safety, efficacy, and quality of these products. This comprehensive framework is vital for anyone involved in clinical research, as it encompasses various categories of biologicals, including human tissues, blood products, and advanced therapies. Understanding these guidelines is not just beneficial; it’s essential for compliance and for navigating TGA's biologicals guidelines in the regulatory process.
Key documents, such as the Australian Regulatory Guidelines for Biologicals (ARGB), outline the requirements for submission, evaluation, and post-market responsibilities. Have you considered how these guidelines impact your research efforts? Recent updates, including the TGA's decision to maintain the current naming system for biologicals and enhance adverse event reporting, are pivotal for research success in Australia. Insights from the International Generic and Biosimilar Medicines Association further reinforce this, indicating that the EU has not identified differences in adverse effects between biosimilars and their reference medicines over the past decade. This underscores the importance of robust regulatory frameworks.
Familiarizing yourself with navigating TGA's biologicals guidelines will provide a solid foundation for your research initiatives in Australia. By understanding the regulatory landscape, you position yourself to navigate challenges effectively and contribute to advancements in the field.
To ensure compliance with TGA's Biologicals Guidelines, it’s essential to gather all necessary documentation, including:
Correctly classifying your product according to TGA standards is crucial, as this classification dictates the specific requirements you must fulfill. Understanding the application process for inclusion in the Australian Register of Therapeutic Goods (ARTG) is vital; this process requires the submission of a comprehensive dossier that substantiates the product's quality, safety, and efficacy.
Engaging with regulatory consultants or legal advisors who specialize in TGA regulations can significantly streamline your preparation efforts. This collaboration not only enhances the likelihood of a successful submission but also provides invaluable insights into navigating TGA's Biologicals Guidelines within the complexities of the regulatory landscape. Notably, the average time for TGA approval of biologicals can vary. However, grasping the nuances of the process will aid in setting realistic timelines for your project.

To implement effective research strategies, it’s crucial to establish a robust study protocol that is focused on navigating TGA biologicals guidelines. This protocol must clearly outline the study design, objectives, methodology, and endpoints. Engaging with knowledgeable research coordinators and investigators who excel in navigating TGA's biologicals guidelines is essential.
Consider employing patient recruitment strategies that tap into diverse patient pools, especially in regions like Latin America and the Balkans, to significantly boost enrollment rates. Consistently tracking trial progress and adherence through established quality assurance procedures is vital, ensuring that all collected data meets the stringent standards required for regulatory submission.
In the evolving Medtech landscape, collaboration is key to overcoming challenges. By leveraging expertise and fostering partnerships, stakeholders can navigate the complexities of clinical research more effectively. As you reflect on your own clinical research challenges, consider how these strategies can enhance your approach and drive successful outcomes.

Navigating the complexities of medical research presents significant challenges, including regulatory compliance, patient recruitment, and data integrity issues. Establishing robust communication channels among all stakeholders - regulatory bodies, sponsors, and research locations - is essential for effectively troubleshooting these obstacles. A proactive approach to patient recruitment can be achieved through targeted outreach and community engagement strategies, which have proven to enhance recruitment efficacy. For instance, the partnership between bioaccess™ and Caribbean Health Group aims to position Barranquilla as a leading site for health studies in Latin America, supported by Colombia's Minister of Health. This initiative exemplifies how innovative partnerships can streamline recruitment processes and improve study outcomes.
Statistics reveal that approximately 80% of clinical trials experience delays due to recruitment issues, with these delays lasting from 1 to 6 months or longer. This highlights the urgent need for timely and effective strategies. Conducting frequent audits and evaluations of data gathering procedures is crucial for navigating TGA's biologicals guidelines and ensuring compliance with INVIMA regulations. INVIMA, Colombia's National Food and Drug Surveillance Institute, oversees medical device regulation and classification. Staying informed about regulatory changes is vital; navigating TGA's biologicals guidelines by subscribing to TGA updates and participating in relevant training sessions can help ensure compliance and adapt to evolving guidelines. Furthermore, bioaccess offers a comprehensive range of services, including:
By implementing these strategies, clinical research organizations can enhance their operational efficiency and improve overall trial outcomes.

Navigating the TGA's Biologicals Guidelines is essential for researchers and stakeholders involved in biological products. Understanding these guidelines not only ensures compliance but also fosters the development of safe and effective therapies. This framework offers the necessary structure to navigate the complexities of biologicals, highlighting the critical importance of adhering to regulatory standards in Australia.
Key aspects vital for compliance include:
Effective clinical research strategies, such as robust study protocols and innovative patient recruitment methods, are crucial for overcoming common challenges in clinical trials. By addressing these elements, researchers can significantly enhance their chances of successful submissions and streamline the approval process.
Ultimately, embracing the TGA's Biologicals Guidelines transcends mere regulatory adherence; it cultivates an environment where quality research can flourish. Stakeholders are urged to actively engage with the guidelines, leverage collaborative opportunities, and stay informed about evolving regulations. By doing so, they contribute to the advancement of medical science while ensuring that patient safety and product efficacy remain at the forefront of their efforts.
What is the role of the Therapeutic Goods Administration (TGA) in Australia regarding biologicals?
The TGA regulates biologicals in Australia, ensuring their safety, efficacy, and quality, which is crucial for clinical research.
What categories of biologicals are covered under TGA's guidelines?
TGA's guidelines encompass various categories of biologicals, including human tissues, blood products, and advanced therapies.
Why is it important to understand TGA's biologicals guidelines?
Understanding these guidelines is essential for compliance and navigating the regulatory process related to biologicals in Australia.
What key documents outline the requirements for biologicals in Australia?
The Australian Regulatory Guidelines for Biologicals (ARGB) outline the requirements for submission, evaluation, and post-market responsibilities.
What recent updates have been made to TGA's biologicals guidelines?
Recent updates include the decision to maintain the current naming system for biologicals and enhance adverse event reporting.
How do the TGA's guidelines impact research efforts?
Familiarizing oneself with the guidelines provides a solid foundation for research initiatives and helps navigate challenges effectively.
What insights have been provided by the International Generic and Biosimilar Medicines Association regarding biosimilars?
Insights indicate that the EU has not identified differences in adverse effects between biosimilars and their reference medicines over the past decade, highlighting the importance of robust regulatory frameworks.