

Navigating the complex landscape of regulatory compliance for Advanced Therapy Medicinal Products (ATMPs) presents significant challenges for many in the pharmaceutical industry. Understanding the Therapeutic Goods Administration (TGA) regulations is crucial for ensuring the safety, efficacy, and timely approval of these innovative therapies.
With evolving guidelines and stringent documentation requirements, stakeholders must ask: how can they effectively streamline their processes and avoid potential pitfalls?
This guide explores the key aspects of TGA regulations, providing insights and strategies designed to enhance compliance and facilitate a smoother path to market for ATMPs.
To effectively navigate how TGA regulates advanced therapy medicinal products (ATMPS), it’s crucial to begin by familiarizing yourself with the Therapeutic Goods Administration (TGA) guidelines. Understanding the following key points will enhance your approach:
By mastering these elements, you’ll be better equipped to navigate how TGA regulates advanced therapy medicinal products (ATMPs), which will ensure a smoother path to approval. What challenges do you face in clinical research that could benefit from this knowledge?
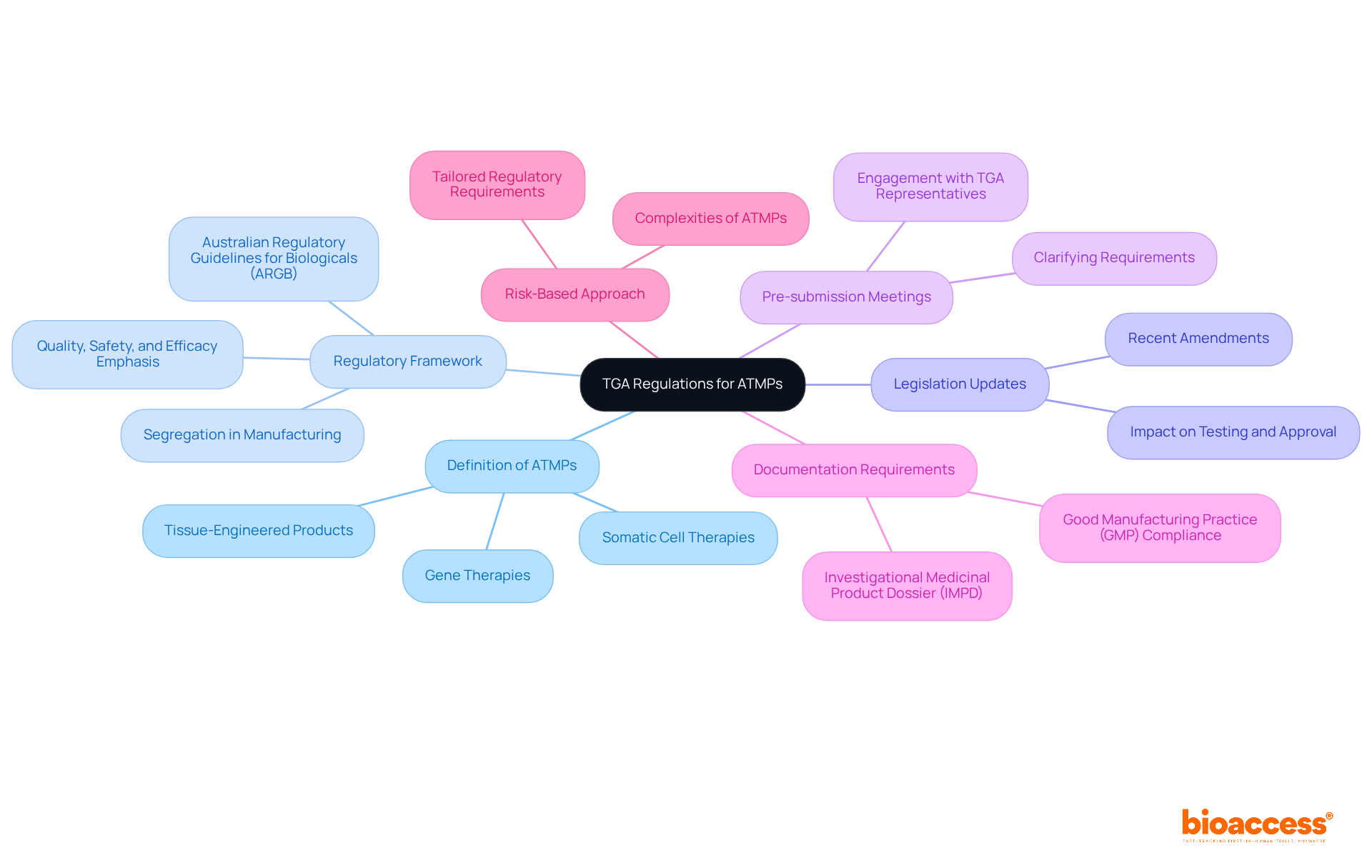
To effectively prepare your submission documents for Advanced Therapy Medicinal Products (ATMPs), follow these essential steps:
Compile Essential Documents: Gather all necessary documentation, including:
Employ the Electronic Common Technical Document (eCTD) Format: The TGA requires submissions in eCTD format, which standardizes document presentation and simplifies the review procedure.
Review and Revise: Conduct comprehensive reviews of all documents to ensure accuracy and completeness. Participating in peer evaluations or consulting with compliance specialists can assist in identifying and correcting any mistakes concerning how TGA regulates advanced therapy medicinal products (ATMPs).
Before finalizing your submission, consider scheduling a pre-submission meeting with TGA officials to learn about how TGA regulates advanced therapy medicinal products (ATMPs). This can clarify any uncertainties regarding your documentation and enhance your submission's quality.
Submission Process: Submit your documents through the TGA’s online portal, ensuring that all files are correctly formatted and labeled.
By meticulously preparing your submission documents, you significantly increase the likelihood of a smooth approval process. Successful case studies, such as those involving companies that navigated complex compliance landscapes, underscore the importance of thorough documentation and adherence to TGA requirements. Following these best practices not only aids in meeting regulations but also prepares your ATMP for timely market entry.
To ensure compliance and effectively monitor the progress of your Advanced Therapy Medicinal Product (ATMP) throughout the regulatory process, consider implementing the following strategies:
Furthermore, utilizing bioaccess's extensive clinical trial management services can greatly improve your adherence efforts. Our expertise in feasibility studies, site selection, regulatory reviews, trial setup, import permits, project management, and reporting ensures that you are well-equipped to navigate the regulatory landscape effectively. By actively managing compliance and monitoring progress, you can facilitate the successful development and commercialization of your ATMP.

Navigating the complexities of TGA regulations for Advanced Therapy Medicinal Products (ATMPs) is not just essential; it’s a critical step toward ensuring compliance and achieving successful market entry. A thorough understanding of TGA guidelines - including definitions, regulatory frameworks, and documentation requirements - empowers stakeholders to manage the approval process effectively. By adopting a proactive approach that incorporates pre-submission meetings and a risk-based strategy, organizations can significantly streamline their path to regulatory approval.
Key insights from this guide underscore the importance of maintaining compliance through:
Establishing a robust regulatory framework and monitoring changes in legislation further enhances the likelihood of success. These practices not only facilitate adherence to TGA requirements but also prepare ATMPs for a timely and efficient market launch.
Ultimately, the journey through TGA regulations for ATMPs demands diligence, knowledge, and strategic planning. By implementing the best practices outlined, stakeholders can navigate this intricate landscape with confidence, ensuring that their innovative therapies reach those in need while upholding the highest standards of safety and efficacy. Engaging with industry resources and staying informed about regulatory developments will further bolster efforts in this vital area of healthcare advancement.
What are advanced therapy medicinal products (ATMPs)?
ATMPs include gene therapies, somatic cell therapies, and tissue-engineered products, each with specific regulatory requirements and unique challenges compared to conventional manufacturing methods.
What is the regulatory framework for ATMPs in Australia?
The regulatory framework for ATMPs in Australia is detailed in the Australian Regulatory Guidelines for Biologicals (ARGB), which outlines evaluation procedures emphasizing quality, safety, and efficacy, along with the need for heightened segregation in manufacturing.
Why is it important to stay informed about legislation updates regarding ATMPs?
Staying informed about recent amendments to the Therapeutic Goods Legislation is essential because new regulatory measures effective from October 2025 may influence testing and approval procedures for ATMPs.
How can pre-submission meetings with TGA representatives be beneficial?
Engaging with TGA representatives early in the process can clarify requirements and expectations, helping to address potential issues before formal submission.
What documentation is required for ATMP submissions?
Necessary documentation includes the Investigational Medicinal Product Dossier (IMPD) and Good Manufacturing Practice (GMP) compliance documents.
What is the importance of a risk-based approach in ATMP regulation?
A risk-based approach allows for tailored regulatory requirements that accommodate the complexities of ATMPs, ensuring that regulations are appropriate for the unique challenges these products present.