


This article examines the various phases of clinical trials for radiopharmaceuticals, emphasizing their critical role in ensuring the safety and efficacy of these specialized drugs in cancer treatment. It delineates each trial phase—from Phase 0 exploratory studies to Phase IV long-term monitoring—underscoring their importance in patient safety, dosage determination, and regulatory compliance. These elements are vital for successful drug development and application in oncology, reinforcing the necessity of thorough clinical evaluation.
Understanding the intricate landscape of radiopharmaceuticals is essential for advancing modern medicine, particularly in oncology. These specialized drugs harness radioactive isotopes for both diagnosis and treatment, positioning themselves at the forefront of targeted therapies that promise to transform patient outcomes. However, navigating the complex phases of clinical trials—each with its own set of challenges and regulatory requirements—can be daunting.
How can researchers ensure success at each checkpoint, from initial feasibility studies to long-term safety monitoring, while effectively recruiting participants and adhering to stringent safety protocols?
Radiopharmaceuticals are specialized drugs containing radioactive isotopes, utilized for both diagnostic and therapeutic purposes. Their dual role is pivotal in modern medicine, particularly in oncology, where they enable precise targeting of cancerous tissues while minimizing damage to surrounding healthy cells. For instance, Iodine-131 (I-131) has been a cornerstone in managing thyroid cancer since the 1940s, effectively targeting residual thyroid tissue and metastatic cells. Similarly, Lutetium-177 (Lu-177) is increasingly recognized for its efficacy in treating neuroendocrine tumors, showcasing the expanding applications of radiopharmaceuticals in clinical settings.
Notably, Pluvicto™ (lutetium Lu 177 vipivotide tetraxetan) represents the first FDA-approved targeted radioligand therapy for advanced prostate cancer, marking a significant advancement in the field. Understanding the mechanisms of action of these agents is essential for study design, as the radiopharmaceuticals trial phases explained directly affect individual safety and results. As the field advances, the significance of radiopharmaceuticals in both cancer care and diagnosis continues to grow, underscoring their importance in the landscape of modern oncology.
Additionally, the substantial rise in clinical studies and research funding in the radiopharmaceutical sector from 2019 to 2024 highlights the increasing significance of this area. This trend stresses the necessity for precise dosimetry in enhancing treatment plans and improving patient outcomes.
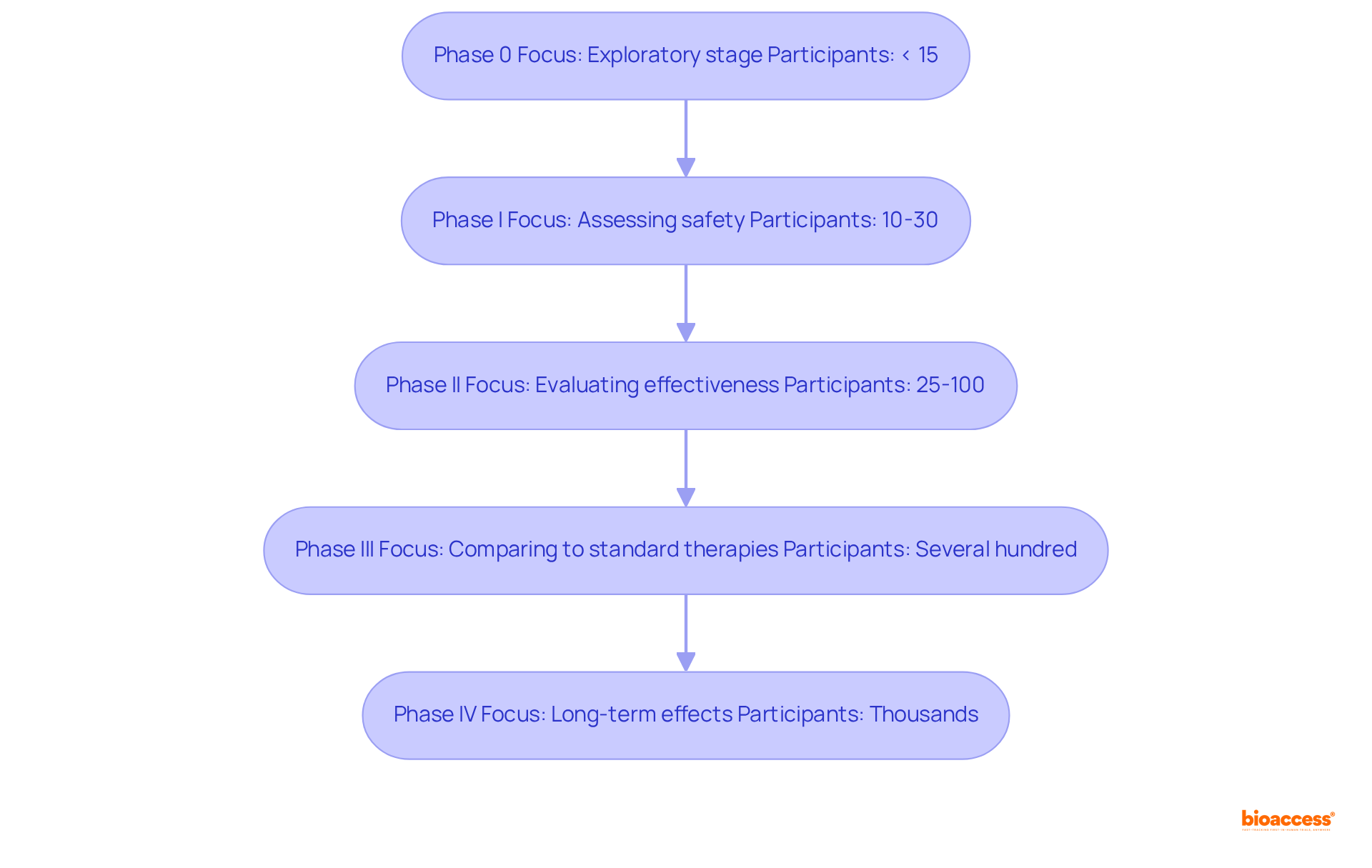
Phase 0 studies serve as an exploratory stage in the radiopharmaceuticals trial phases explained, focusing on pharmacokinetics and pharmacodynamics with a limited number of participants, typically fewer than 15. These experiments aim to establish feasibility and enhance biomarker assay techniques prior to initiating more extensive studies. The National Cancer Institute (NCI) underscores the significance of this phase in evaluating the biological activity of new treatments, particularly for rare diseases with restricted patient populations.
The radiopharmaceuticals trial phases explained indicate that Phase I studies primarily concentrate on assessing safety and determining the highest tolerated dose of the radiopharmaceutical in healthy participants. Generally involving 10 to 30 participants, these studies are critical for identifying potential side effects and establishing safe dosage levels. Successful Phase I trials yield vital data that inform the radiopharmaceuticals trial phases explained, which ensures that the intervention is both safe and effective.
In the context of radiopharmaceuticals trial phases explained, Phase II shifts the focus to evaluating the effectiveness of the intervention within a broader cohort, typically comprising 25 to 100 individuals with the same type of cancer. This phase assesses whether the intervention can reduce tumors, prolong progression-free survival, and improve quality of life. Dose optimization is also a crucial aspect, as researchers strive to pinpoint the safest and most effective dose based on Phase I findings.
The safety and efficacy of the new radiopharmaceutical are assessed in Phase III studies, as outlined in the radiopharmaceuticals trial phases explained, which compare it to standard therapies across a diverse group, often including several hundred patients. These experiments may utilize random allocation to ensure unbiased outcomes and are essential for determining whether the new intervention should be approved for widespread use.
Finally, the long-term effects of the intervention and additional data on its effectiveness and safety are monitored in Phase IV studies, as part of the radiopharmaceuticals trial phases explained. Typically involving thousands of participants, these studies are crucial for understanding the treatment's impact over time, including rare side effects that may not have been apparent in earlier phases.
With bioaccess's expertise in managing research studies, including Early-Feasibility Studies and First-In-Human Studies, the process can be accelerated through the 'Patient Velocity' feature, achieving patient cohorts 50% faster and saving $25K per patient. This approach directly addresses common recruitment challenges faced in early-stage studies, while bioaccess's comprehensive management services ensure a seamless process from feasibility assessments to post-market follow-up.
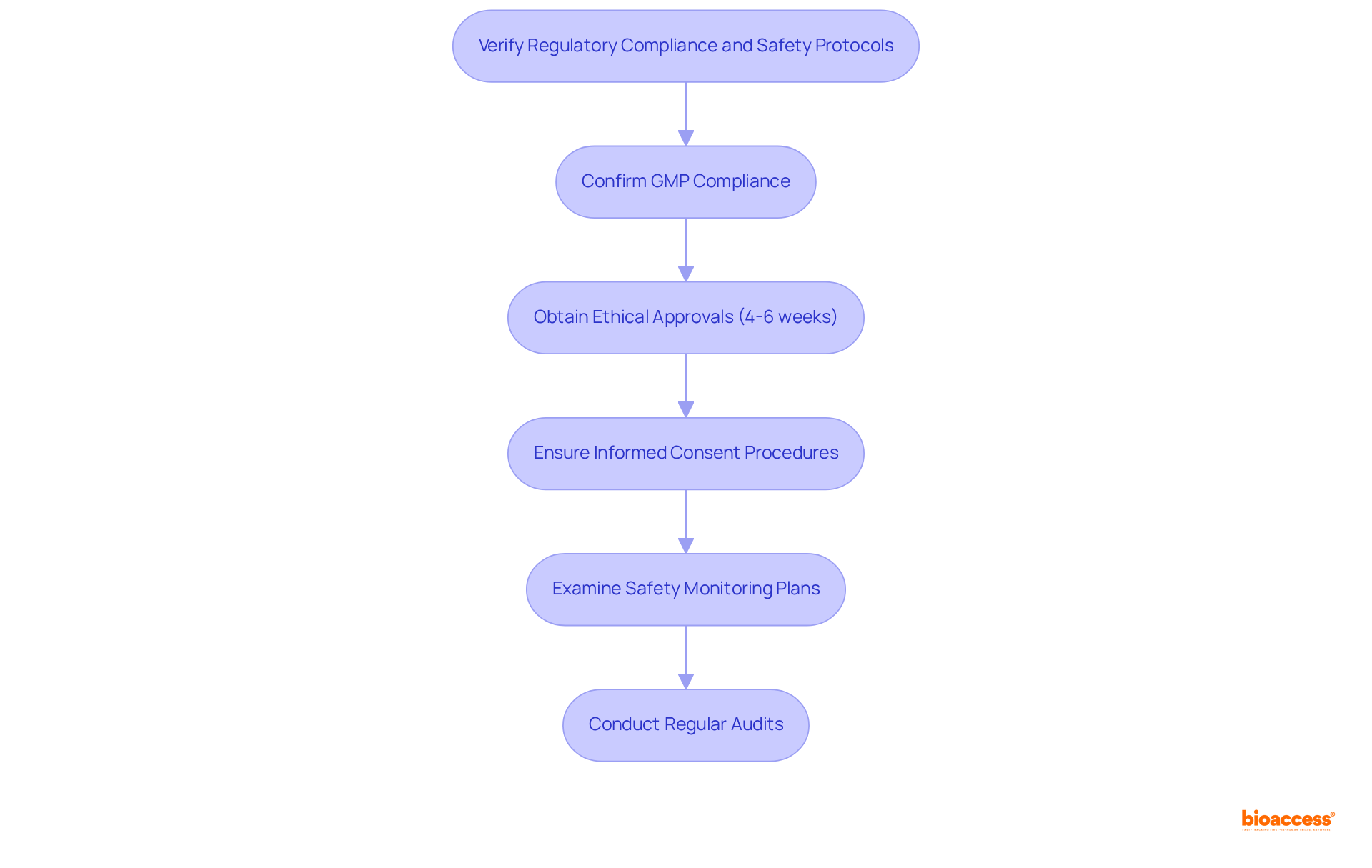
It is imperative to confirm that all radiopharmaceuticals are manufactured in strict accordance with Good Manufacturing Practices (GMP), which are essential for ensuring safety, quality, and efficacy throughout the production process. Timely approval of all study protocols by relevant ethics committees (IRB/EC) and regulatory authorities, including INVIMA and the Ministry of Health, is crucial, with an average timeline of 4-6 weeks for ethical approvals, facilitating a seamless progression through the clinical research phases.
Furthermore, it is vital to ensure that informed consent procedures are robust and clearly communicated to participants, safeguarding their rights and ensuring they fully understand the implications of the study. A thorough examination of safety monitoring plans is necessary, including strategies for adverse event reporting and management, to swiftly address any safety concerns that may arise during the study. This includes leveraging data from previous audits, which have demonstrated an average reliability of 84% in the production process.
Regular audits must be performed to guarantee continuous adherence to all regulatory mandates, thereby strengthening the integrity of the study and the safety of participants. Notably, productivity at the production site has significantly increased from 2 to 27 productions per month over a 21-month period, showcasing the effectiveness of these compliance measures.
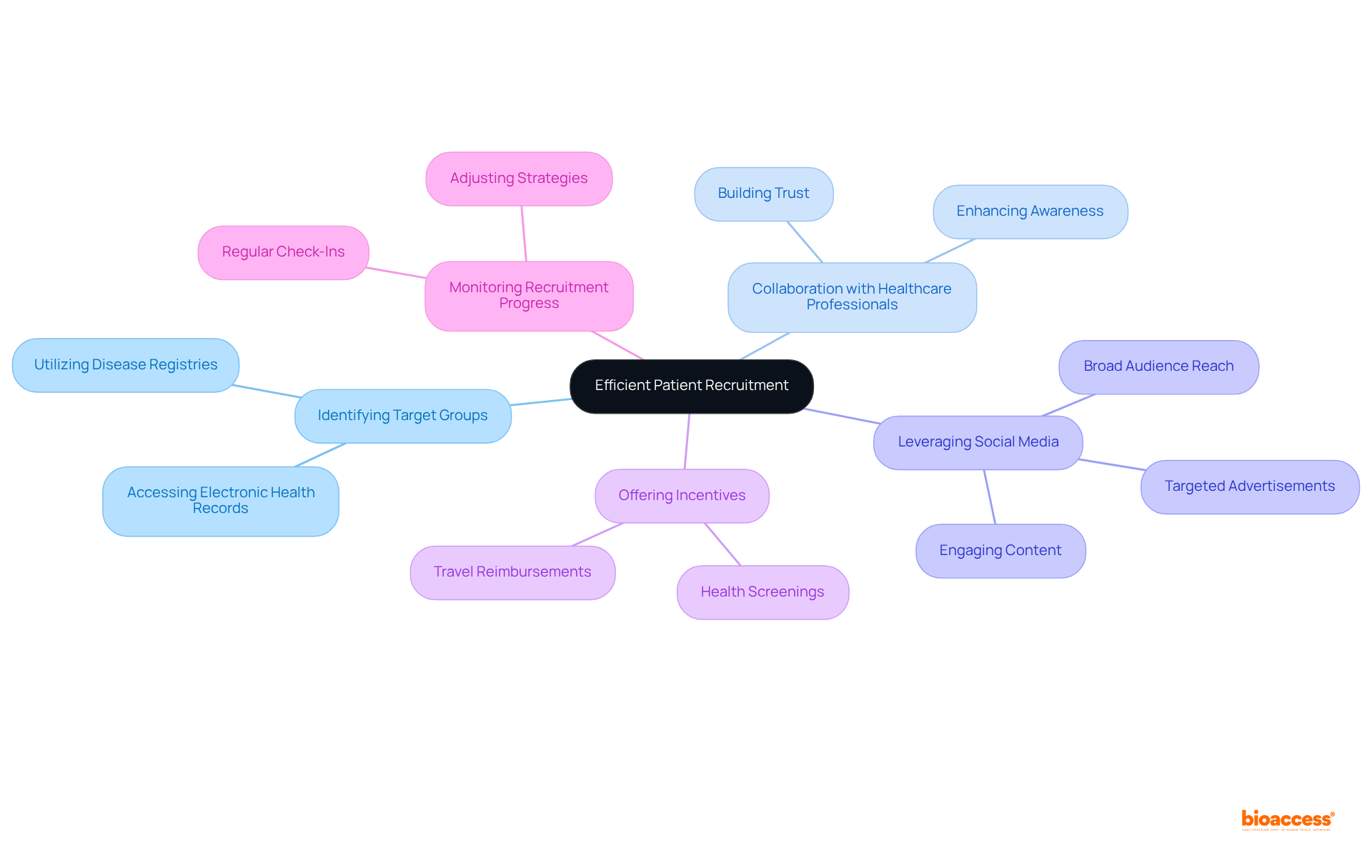
Identifying target groups through disease registries and electronic health records is essential for simplifying the recruitment process, as these resources provide access to individuals who meet specific study criteria.
Collaboration with healthcare professionals is crucial; their established connections can enhance awareness of the study, effectively conveying the advantages and significance of participation.
Furthermore, leveraging social media and online platforms allows for outreach to a broader audience, engaging potential participants through targeted advertisements and informative content. With over 3 billion individuals actively engaging on platforms such as Facebook and Instagram, these channels significantly enhance awareness and interest in research studies.
To encourage enrollment and demonstrate appreciation for participants' time and commitment, offering incentives such as travel reimbursements or health screenings is advisable.
Regular monitoring of recruitment progress is necessary, enabling adjustments to strategies as challenges arise. This proactive approach is vital, given that more than half of ongoing clinical trials struggle with patient recruitment; timely adjustments can mitigate delays that may lead to budget overruns or trial failures.
In conclusion, radiopharmaceuticals represent a pivotal advancement in modern medicine, especially within oncology, where they enable targeted treatment strategies that minimize damage to healthy tissues. A comprehensive understanding of the clinical trial phases for radiopharmaceuticals is paramount, as each stage acts as a critical checkpoint ensuring safety, efficacy, and adherence to regulatory standards. This methodical approach not only increases the probability of successful outcomes but also underscores the vital role these innovative therapies play in enhancing patient care.
The article has meticulously explored the phases of radiopharmaceutical clinical trials, emphasizing the importance of each stage—from Phase 0 exploratory studies to Phase IV long-term monitoring. Notable insights include the essential nature of safety evaluations in Phase I, the effectiveness assessments in Phase II, and the comparative analyses in Phase III, all of which are instrumental in guiding informed decision-making for future treatments. Furthermore, the significance of regulatory compliance and effective patient recruitment strategies has been highlighted as crucial elements in navigating the complexities inherent in clinical trials.
Ultimately, the progress made in radiopharmaceuticals heralds a transformative evolution in cancer treatment and diagnostics. As research continues to advance, it is crucial for stakeholders within the healthcare sector to prioritize efficient trial designs, adhere to safety protocols, and actively engage with potential participants. By embracing these strategies, the field can fully realize the potential of radiopharmaceuticals, paving the way for groundbreaking therapies that not only enhance treatment efficacy but also significantly improve the quality of life for patients confronted with challenging diagnoses.
What are radiopharmaceuticals?
Radiopharmaceuticals are specialized drugs that contain radioactive isotopes and are used for both diagnostic and therapeutic purposes in medicine.
What role do radiopharmaceuticals play in clinical trials?
Radiopharmaceuticals are pivotal in clinical trials, particularly in oncology, as they enable precise targeting of cancerous tissues while minimizing damage to surrounding healthy cells.
Can you provide an example of a radiopharmaceutical used in cancer treatment?
Iodine-131 (I-131) has been a key treatment for thyroid cancer since the 1940s, effectively targeting residual thyroid tissue and metastatic cells.
What is Pluvicto™ and its significance?
Pluvicto™ (lutetium Lu 177 vipivotide tetraxetan) is the first FDA-approved targeted radioligand therapy for advanced prostate cancer, representing a significant advancement in the use of radiopharmaceuticals.
Why is understanding the mechanisms of action of radiopharmaceuticals important?
Understanding the mechanisms of action is crucial for study design in clinical trials, as it directly affects individual safety and the results of the trials.
What trends are observed in the radiopharmaceutical sector from 2019 to 2024?
There has been a substantial rise in clinical studies and research funding in the radiopharmaceutical sector, indicating its increasing significance in cancer care and diagnosis.
What is the importance of dosimetry in radiopharmaceuticals?
Precise dosimetry is necessary for enhancing treatment plans and improving patient outcomes in the use of radiopharmaceuticals.