


Establishing a Data Monitoring Board (DMB) under TGA regulations is essential for ensuring the safety and effectiveness of clinical trials. This guide provides a thorough, step-by-step approach to assembling a skilled team, setting up compliance protocols, and defining operational procedures that uphold the integrity of medical research. As the landscape of clinical trials continues to evolve, researchers must consider how to ensure their DMB not only meets regulatory standards but also adapts to emerging challenges while maintaining participant trust.
In this dynamic environment, the role of a DMB becomes increasingly significant. It’s not just about compliance; it’s about fostering a culture of safety and transparency. By understanding the key challenges in the Medtech landscape, researchers can better position their DMBs to respond effectively. This guide will delve into the strategies that can help navigate these complexities, ensuring that clinical trials remain robust and trustworthy.
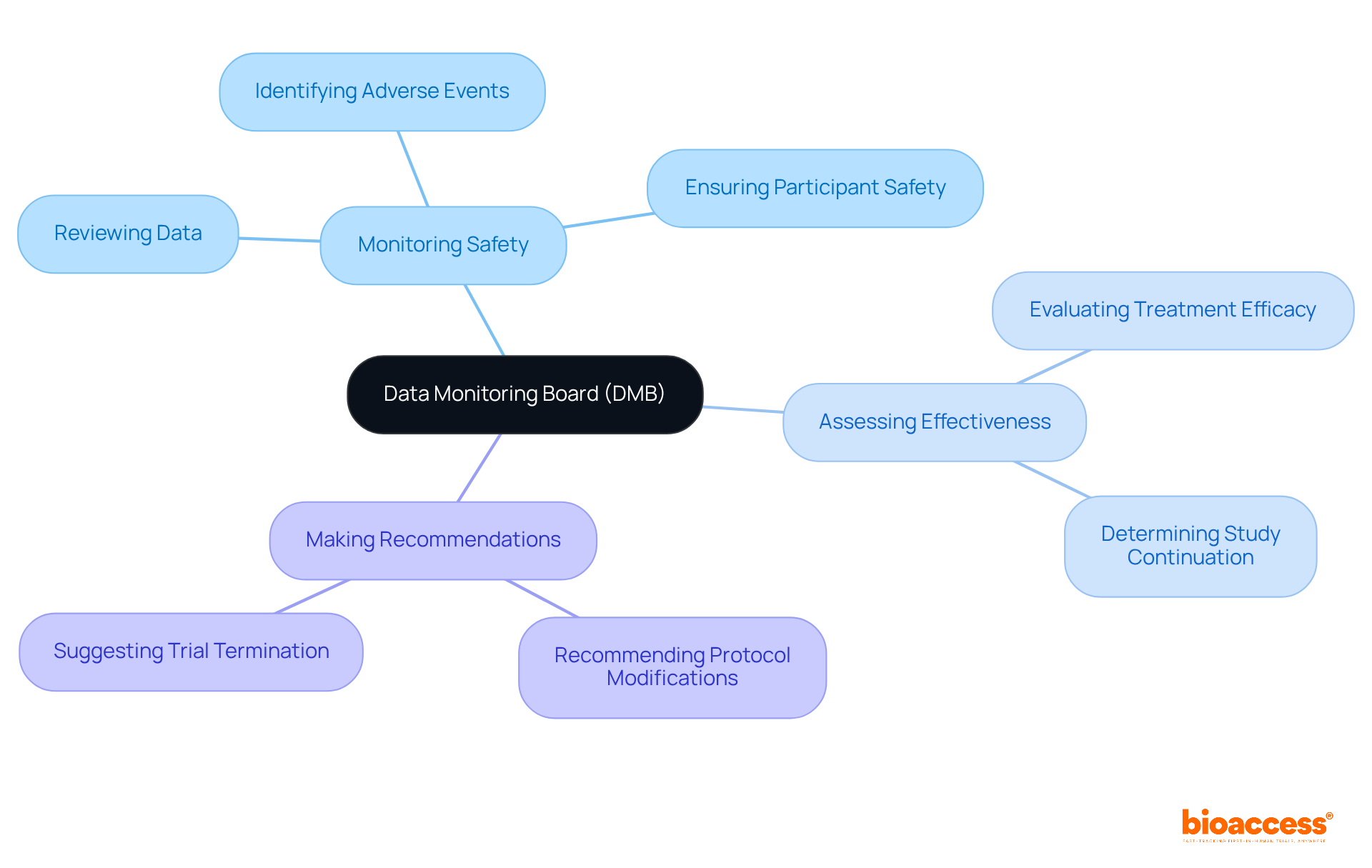
A Data Monitoring Board (DMB) is an autonomous organization established to oversee the safety and effectiveness of clinical studies, specifically through the data monitoring board setup under TGA regulations. The DMB plays a pivotal role in several key areas:
The significance of a data monitoring board setup under TGA in regulated studies cannot be overstated. By providing independent oversight, DMBs enhance the credibility of medical research and ensure compliance with ethical standards. For instance, in recent experiments, DMBs have successfully identified safety issues early, leading to essential modifications that protected participant health and preserved the integrity of the study. As medical research continues to evolve in 2025, the role of DMBs remains crucial in fostering trust and ensuring that innovative treatments are both safe and effective.
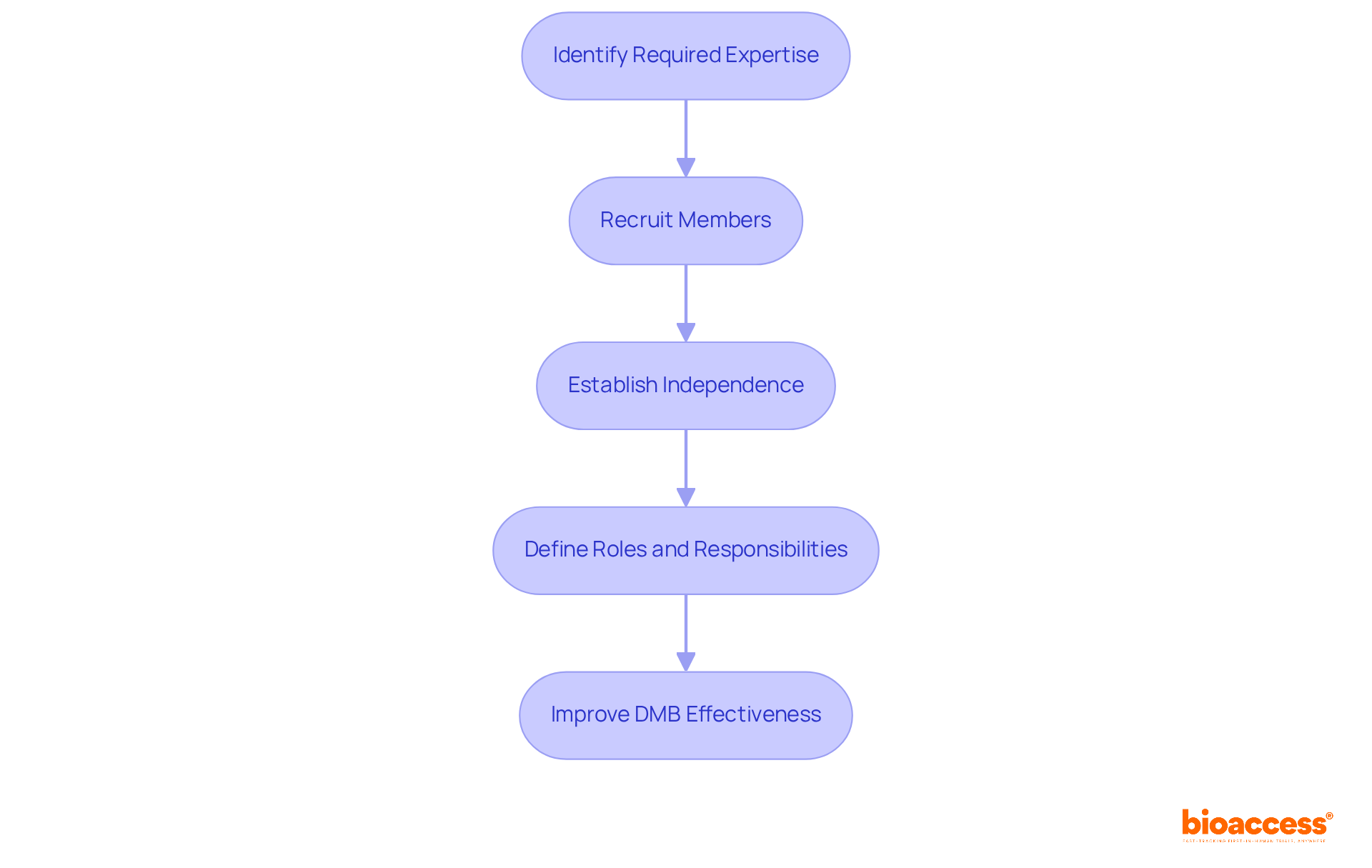
To assemble an effective Data Monitoring Board (DMB), it’s crucial to follow these essential steps:
Identify Required Expertise: Determine the necessary qualifications for DMB members, focusing on medical, statistical, and ethical knowledge pertinent to the study. Members should possess a deep understanding of the therapeutic area and the regulatory landscape.
Recruit members who have a proven track record in clinical research and are familiar with the data monitoring board setup under TGA. Ideal candidates include:
Establish Independence: Guarantee that DMB members are autonomous from the sponsor. This independence is crucial for preserving objectivity in their evaluations and suggestions, thereby protecting the integrity of the examination.
Define Roles and Responsibilities: Clearly outline the roles and responsibilities of each member to prevent overlaps and ensure accountability. This clarity helps streamline decision-making processes and enhances the DMB's overall effectiveness.
By thoughtfully choosing a varied and skilled team, you can greatly improve the DMB's ability to oversee trial integrity and effectiveness. This ultimately aids the success of your clinical research initiatives.
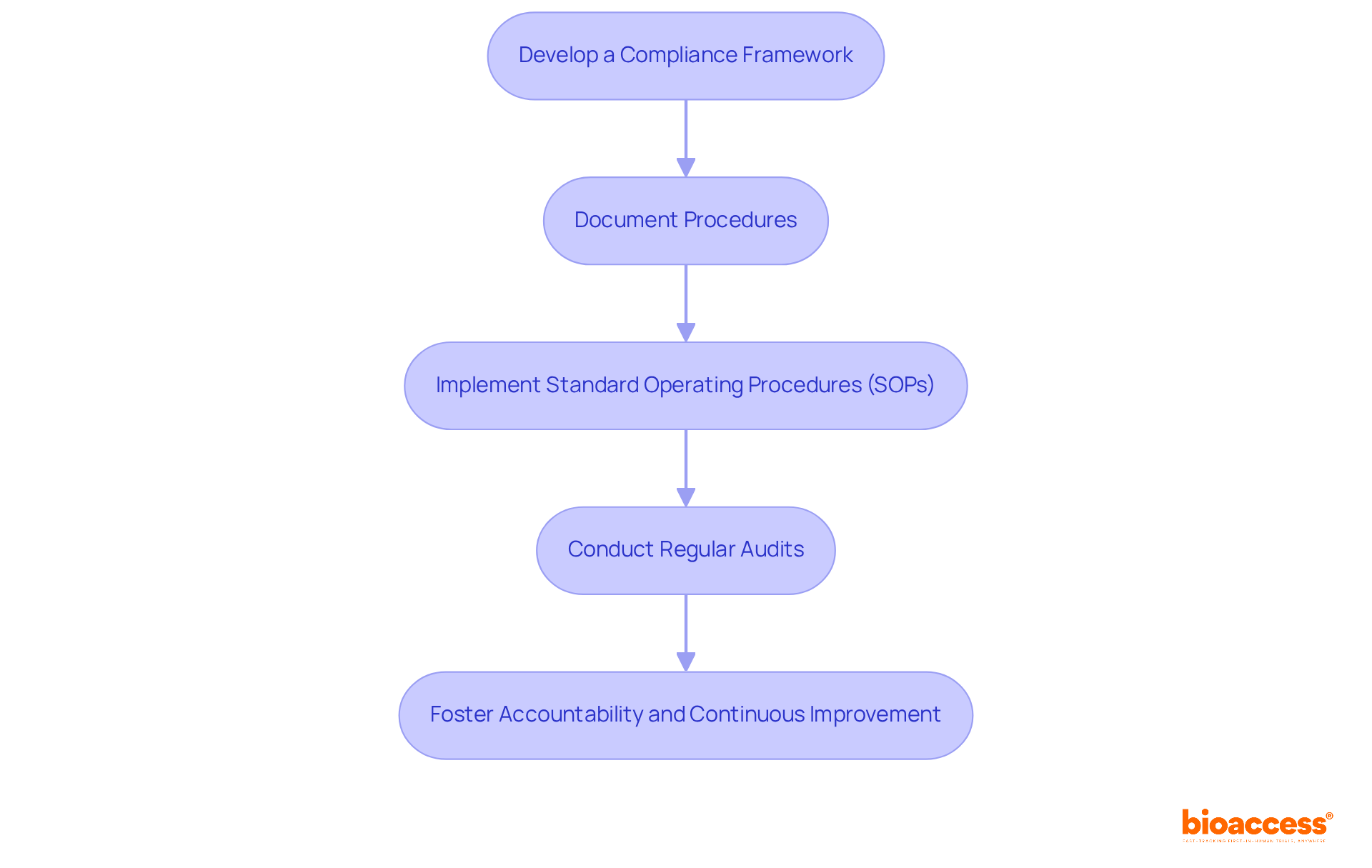
To establish effective compliance and documentation procedures for your Data Monitoring Board (DMB), it’s crucial to follow these essential steps:
Develop a Compliance Framework: Clearly outline the regulatory requirements mandated by the TGA for the data monitoring board setup under TGA operations. This ensures clarity on compliance expectations and sets the foundation for accountability.
Document Procedures: Meticulously document all procedures related to data monitoring, risk evaluations, and reporting. This should encompass:
Implement Standard Operating Procedures (SOPs): Create detailed SOPs that govern DMB operations. These should include data handling protocols, confidentiality agreements, and communication strategies to safeguard sensitive information.
The data monitoring board setup under TGA will be crucial for overseeing the project. Conduct regular audits of the data monitoring board setup under TGA to verify adherence to established procedures and regulations. This fosters a culture of accountability and continuous improvement.
By implementing these structured procedures, your DMB will not only operate within the legal framework but also maintain high standards of accountability and security.
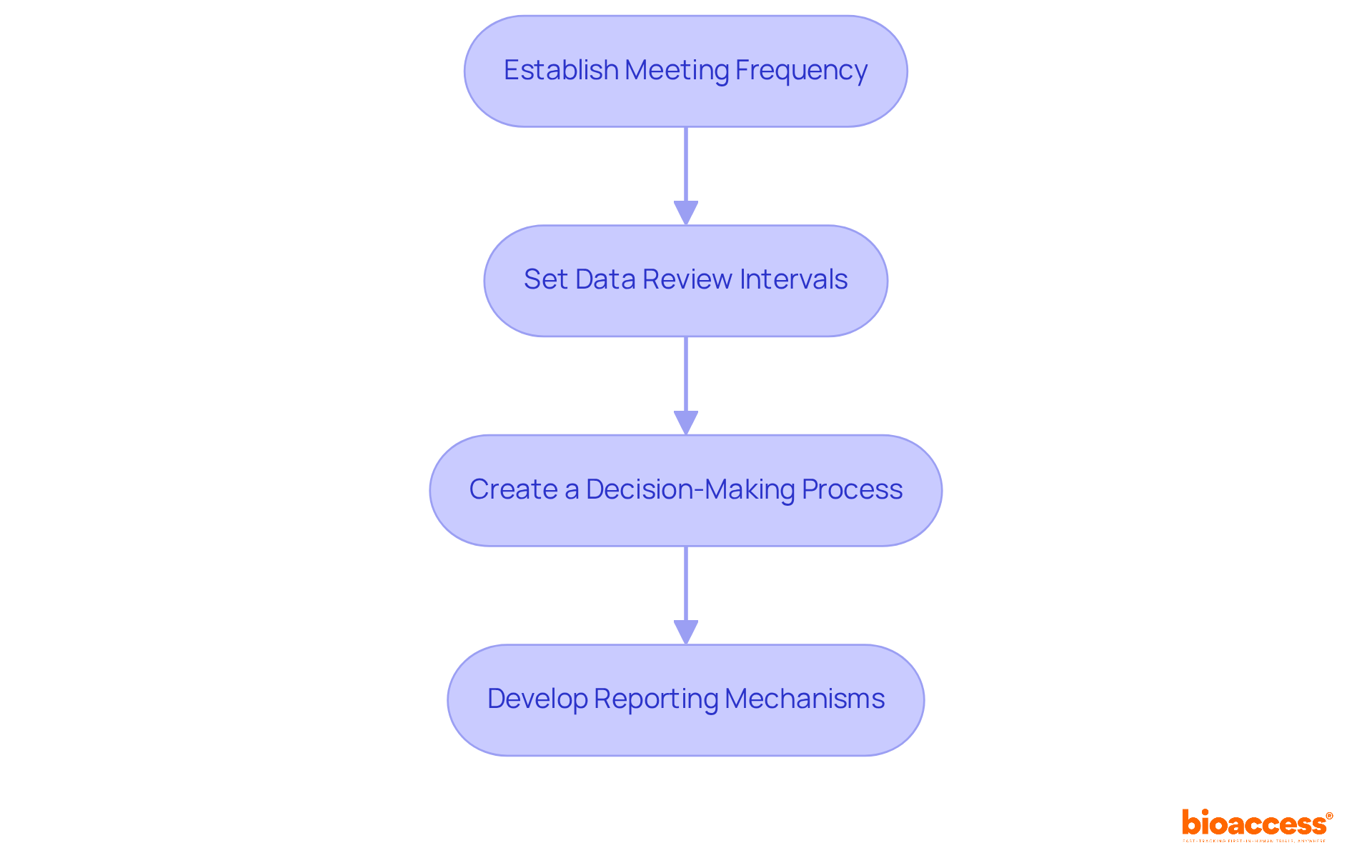
To define operational protocols for your Data Monitoring Board (DMB), it’s essential to follow these guidelines:
By clearly defining these operational protocols, you can significantly enhance the data monitoring board setup under TGA's effectiveness in monitoring clinical trials and safeguarding participant welfare.
Establishing a Data Monitoring Board (DMB) under the TGA is not just a procedural step; it’s a pivotal move in safeguarding the safety and efficacy of clinical trials. This guide has laid out the essential components for setting up a DMB, underscoring the critical role of independent oversight in upholding ethical standards and ensuring participant welfare throughout the research process.
Key steps involve:
Each of these elements is vital in enhancing the DMB's effectiveness, enabling it to monitor safety, assess treatment efficacy, and make informed recommendations based on thorough data analysis.
Ultimately, the establishment of a robust Data Monitoring Board under TGA regulations not only strengthens the integrity of clinical trials but also cultivates trust within the research community. It is imperative for stakeholders to prioritize these guidelines, ensuring that innovative treatments are developed safely and responsibly. This commitment paves the way for advancements in medical research and improved patient outcomes.
What is a Data Monitoring Board (DMB)?
A Data Monitoring Board (DMB) is an autonomous organization established to oversee the safety and effectiveness of clinical studies, specifically under TGA regulations.
What are the primary responsibilities of a DMB?
The primary responsibilities of a DMB include monitoring participant safety, assessing the effectiveness of treatments, and making recommendations regarding study protocols based on their findings.
How does the DMB monitor safety in clinical studies?
The DMB systematically reviews accumulating data to ensure participant safety and uphold the integrity of the trial, enabling prompt recognition of any adverse incidents or concerns.
In what ways does the DMB assess the effectiveness of treatments?
The DMB evaluates the treatment's efficacy by determining whether the study should proceed based on the data collected, making informed decisions regarding the continuation of the experiment.
What actions can a DMB take based on their findings?
A DMB can recommend modifications to the study protocol or, in severe cases, terminate the study if significant safety issues are identified.
Why is the role of a DMB significant in regulated studies?
The role of a DMB is significant because it provides independent oversight, enhances the credibility of medical research, and ensures compliance with ethical standards.
How have DMBs impacted recent clinical experiments?
DMBs have successfully identified safety issues early in recent experiments, leading to essential modifications that protected participant health and preserved the integrity of the study.
What is the importance of DMBs as medical research evolves?
As medical research continues to evolve, the role of DMBs remains crucial in fostering trust and ensuring that innovative treatments are both safe and effective.