

This report outlines the strategic imperative for Brazil's leading healthcare institutions to invest in vertically integrated, in-house theranostics programs, complete with a medical cyclotron and radiopharmacy. It addresses the critical knowledge gap that views this infrastructure as a prohibitive cost, reframing it as a powerful engine for revenue generation, market leadership, and enhanced patient care. The analysis details the clinical revolution of theranostics, exposes the vulnerabilities of Brazil's current reliance on imported radioisotopes, and provides a financial blueprint for achieving profitability through diversified revenue streams. Ultimately, it serves as a call to action for institutions like Hospital Israelita Albert Einstein to seize a first-mover advantage, secure operational sovereignty, and define the future of advanced cancer care in Latin America.
The field of oncology is undergoing a paradigm shift, moving from generalized treatments to the era of precision medicine. At the apex of this evolution is theranostics, a revolutionary approach that integrates diagnosis and therapy into a single, targeted platform, offering personalized and highly effective treatments for cancer. This is not an incremental improvement but a fundamental redefinition of how cancer care is delivered. For Brazil's leading healthcare institutions, embracing this shift is not merely an option but a strategic imperative for future relevance and leadership.
However, a critical strategic vulnerability currently constrains Brazil's potential in this domain. The nation's healthcare system, including its most advanced private hospitals, remains heavily dependent on imported and centrally distributed radioisotopes. This reliance creates significant logistical barriers, introduces supply chain fragility, and ultimately limits patient access to the most advanced forms of care. This dependency represents a severe handicap for any institution aspiring to regional and international prominence.
This report addresses a crucial knowledge gap prevalent among healthcare executives: the mischaracterization of an in-house medical cyclotron and integrated radiopharmacy as a prohibitive capital expense. This perspective is a critical miscalculation. The true knowledge gap is financial and strategic. A vertically integrated theranostics program, powered by an on-site cyclotron, is not a cost center but a powerful platform for market differentiation. It is an engine for creating multiple, high-margin revenue streams and establishing indisputable regional leadership in the most advanced and profitable sector of modern oncology.
The investment thesis presented herein is clear: for a premier institution such as Hospital Israelita Albert Einstein, the investment in an on-site cyclotron and a Good Manufacturing Practices (GMP)-compliant radiopharmacy is not only financially viable but strategically essential. Such an investment unlocks operational sovereignty, shielding the institution from external supply chain disruptions. It dramatically expands clinical capabilities far beyond competitors by enabling the use of a wide array of novel diagnostic and therapeutic agents. Most importantly, it generates a compelling return on investment through a diversified business model that includes enhanced clinical services, commercial radiopharmaceutical distribution, and high-value clinical research partnerships.
The time for incrementalism is over. The competitive landscape of advanced healthcare is being redrawn by institutions that control their own technological destiny. This report serves as a call to action for Brazil's healthcare leaders. It is time to consider a bold, legacy-defining investment that will not only transform patient care for a generation but also secure their institution's position at the vanguard of medicine in Latin America for decades to come.
The convergence of molecular biology, nuclear physics, and oncology has given rise to theranostics, a discipline that is fundamentally altering the approach to cancer treatment. Its core principle—"see what you treat, treat what you see"—represents the ultimate realization of personalized medicine, moving beyond the one-size-fits-all models of traditional chemotherapy and external beam radiation towards a future where treatment is tailored to the unique molecular signature of each patient's disease.
Theranostics achieves its precision by using a single molecular agent designed for two complementary purposes. The term itself is a portmanteau of "therapy" and "diagnostics". The process involves a targeting molecule, such as an antibody or peptide, that is engineered to bind to a specific receptor or protein found on the surface of cancer cells. This targeting molecule is then paired with a radioactive isotope. For the diagnostic phase, a low-energy, image-emitting radioisotope like Gallium-68 (
68Ga) is attached. When administered to a patient, this radiopharmaceutical travels through the body and accumulates at the tumor sites, allowing physicians to visualize the exact location and extent of the cancer with a Positron Emission Tomography (PET) scan.
If the diagnostic scan confirms the presence and accessibility of the target, the therapeutic phase can commence. The same targeting molecule is then paired with a different, high-energy, cell-destroying radioisotope, such as Lutetium-177 (177Lu) or Actinium-225 (225Ac). This therapeutic radiopharmaceutical is administered and, following the same biological pathway, delivers a potent dose of radiation directly to the cancer cells with surgical precision. This targeted delivery mechanism is a key advantage over conventional treatments; it maximizes the destructive force on the tumor while minimizing collateral damage to surrounding healthy tissues, thereby reducing the severe side effects often associated with systemic therapies.
This powerful pairing of agents eliminates the uncertainty inherent in many traditional cancer treatments. It provides a definitive, personalized pathway, ensuring that only patients whose tumors express the specific molecular target—and are therefore likely to benefit—receive the therapy. This approach optimizes clinical outcomes, avoids the toxicity and cost of ineffective treatments, and provides patients and their families with a clearer understanding of their therapeutic options.
The implementation of a successful theranostics program is an inherently multidisciplinary endeavor. It requires the seamless integration and collaboration of a specialized team, including nuclear medicine physicians, medical oncologists, radiopharmacists, medical physicists, radiation safety officers, and highly trained nursing staff. This collaborative structure fosters a culture of integrated, high-level care, breaking down traditional departmental silos and creating a patient-centric service line that is greater than the sum of its parts. An institution that builds such a program is not merely adding a new technology; it is cultivating a new, more advanced model of patient care that attracts top-tier medical talent. These experts are drawn to environments where they can practice at the forefront of their field, further enhancing the institution's intellectual capital and reputation for excellence. This "halo effect" elevates the entire oncology service line, making the hospital a magnet for complex cases, innovative research, and the next generation of medical leaders.
The promise of theranostics is not theoretical; it is a clinical reality that is already transforming patient outcomes in some of the most challenging areas of oncology. The rapid adoption and success of theranostic agents for prostate cancer and neuroendocrine tumors (NETs) serve as powerful case studies demonstrating the profound impact of this approach.
Prostate Cancer Case Study (PSMA): The management of metastatic castration-resistant prostate cancer (mCRPC), a stage of the disease where tumors no longer respond to hormone therapy, has been revolutionized by theranostics targeting the Prostate-Specific Membrane Antigen (PSMA). PSMA is a protein that is highly overexpressed on the surface of most prostate cancer cells. The theranostic pair consists of a diagnostic agent, such as
68Ga-PSMA-11, used for PET imaging, and a therapeutic agent, 177Lu-PSMA-617 (commercially known as Pluvicto™), for treatment.
The clinical evidence is compelling. The landmark VISION trial demonstrated that patients with mCRPC treated with 177Lu-PSMA-617 plus standard of care had significantly longer overall survival (a median of 15.3 months) compared to those receiving standard of care alone (11.3 months). Furthermore, studies, including those involving Brazilian patients, have shown that this therapy can lead to dramatic reductions in Prostate-Specific Antigen (PSA) levels—a key biomarker for the disease—and marked improvements in quality of life, often for patients who had exhausted all other available treatment options. One Brazilian startup, supported by FAPESP, has developed a local formulation of PSMA that has reduced treatment costs by over 50%, yet even with this progress, access remains extremely limited, reaching less than 1% of eligible patients in the country, highlighting a massive unmet need.
Neuroendocrine Tumors (NETs) Case Study (SSTRs): Similarly, theranostics has become a cornerstone in the treatment of well-differentiated, advanced NETs, which are characterized by the overexpression of somatostatin receptors (SSTRs). The established theranostic pair for this disease is
68Ga-DOTATATE for PET imaging and 177Lu-DOTATATE (Lutathera™) for therapy. The pivotal NETTER-1 trial showed that patients treated with Lutathera™ had a 79% lower risk of disease progression or death compared to those treated with high-dose octreotide, the previous standard of care. This therapy not only halts tumor growth but also significantly improves patients' quality of life by controlling the debilitating symptoms often associated with these hormone-secreting tumors. Importantly, studies conducted in Brazil have confirmed these positive outcomes, demonstrating the treatment's effectiveness and low toxicity profile within the local patient population.
The Expanding Pipeline: The success in prostate cancer and NETs is just the beginning. The theranostics field is rapidly expanding, with a robust pipeline of new agents targeting a wide range of malignancies. Clinical trials are actively exploring its potential in melanoma, breast cancer, pancreatic cancer, and other hard-to-treat cancers. A particularly promising area of research involves targeting Fibroblast Activation Protein (FAP), a protein found in the stroma of a majority of solid tumors. FAP-targeted agents are being investigated as a potential "pan-cancer" theranostic, capable of diagnosing and treating many different types of cancer with a single molecular platform. For a leading healthcare institution, being positioned to participate in and adopt these future breakthroughs is not just an opportunity but a necessity for maintaining a competitive edge in oncology.
While the clinical promise of theranostics is undeniable, its widespread adoption in Brazil is severely hampered by a fundamental, structural weakness: a deep-seated reliance on a fragile and geographically constrained radiopharmaceutical supply chain. This dependence creates significant operational hurdles, limits patient access to care, and represents a critical strategic vulnerability for any healthcare institution aiming for leadership. Understanding this dilemma is the first step toward recognizing the profound opportunity that awaits a forward-thinking organization.
Brazil's nuclear medicine sector is substantial, performing approximately 2 million procedures annually. However, the vast majority of these procedures rely on radioisotopes that are produced in nuclear reactors and imported from a handful of international suppliers. This dependency was starkly exposed in 2009 when the unexpected shutdown of a major Canadian research reactor triggered a global supply crisis of Molybdenum-99 (
99Mo). As
99Mo is the parent isotope of Technetium-99m (99mTc), the workhorse radioisotope for over 80% of all nuclear medicine diagnostic procedures, the shortage had a crippling effect on services worldwide, including in Brazil. This event served as a powerful lesson in the risks of a centralized, import-dependent supply chain and was a primary catalyst for the Brazilian government's long-term plan to construct the Brazilian Multipurpose Reactor (RMB) to achieve national self-sufficiency in reactor-based isotopes.
The supply chain challenge is magnified exponentially for the radioisotopes required for modern PET imaging and theranostics. Unlike reactor-produced isotopes that may have half-lives of several days, the most crucial PET isotopes are produced in particle accelerators called cyclotrons and have extremely short half-lives. Fluorine-18 (18F), the isotope used in the vast majority of PET scans, has a half-life of approximately 110 minutes. Gallium-68 (68Ga), a key diagnostic partner for many theranostic therapies, has a half-life of just 68 minutes.
These physical properties create an inescapable logistical reality: these isotopes cannot be stockpiled or transported over long distances. They must be produced in close proximity to where they will be used, typically within a two-hour transport radius. This has resulted in a stark geographical disparity in the availability of advanced medical care in Brazil. The country's few medical cyclotrons are heavily concentrated in the Southeast region. Consequently, access to advanced PET diagnostics, and by extension the entire field of theranostics, is effectively restricted to patients living in or near major metropolitan centers like São Paulo and Rio de Janeiro, leaving vast portions of the North, Northeast, and Central-West regions critically underserved. For a patient in these regions, the availability of state-of-the-art cancer care is dictated not by their clinical need or the expertise of their physicians, but by their postal code. This logistical barrier means that for most of Brazil, the theranostics revolution remains a distant promise, creating a significant and growing gap in healthcare equity and innovation.
A common misconception within the Brazilian healthcare landscape is that the forthcoming Brazilian Multipurpose Reactor (RMB) will solve the nation's radiopharmaceutical supply issues. While the RMB is a vital strategic project that will eventually provide national self-sufficiency for reactor-based isotopes like 99Mo and the therapeutic isotope 177Lu, it is crucial to understand its limitations. The RMB is a nuclear reactor, not a cyclotron. It
will not produce the short-lived, positron-emitting isotopes (18F, 68Ga, 11C, etc.) that are the essential foundation of modern molecular imaging and the diagnostic component of theranostics.
This distinction represents the central knowledge gap that must be addressed. Relying on the RMB, which is still at least five years from operation, as a solution for advancing a hospital's theranostics capabilities is a flawed strategy. The RMB addresses the vulnerabilities of the past (the
99Mo crisis) but does not provide the tools required for the medicine of the future. This reality creates a clear, urgent, and highly valuable opportunity for private institutions to step into this technological void. By investing in their own cyclotron capabilities, they can leapfrog the current limitations and position themselves as the primary enablers of advanced cancer care, long before the national infrastructure catches up.
This situation gives rise to a "Vulnerability Paradox" in Brazilian healthcare: the more clinically advanced and effective a radiopharmaceutical is, the more likely it is to have a short half-life, and therefore, the less accessible it is to the broader population under the current infrastructure. As nuclear medicine continues to innovate globally with even more sophisticated, shorter-lived agents, this access gap will only widen. An institution that invests in its own cyclotron does not merely catch up; it fundamentally breaks this paradox. It gains the sovereign capability to produce and deploy the most advanced agents immediately, creating a powerful and durable competitive advantage that is logistically impossible for dependent competitors to overcome.
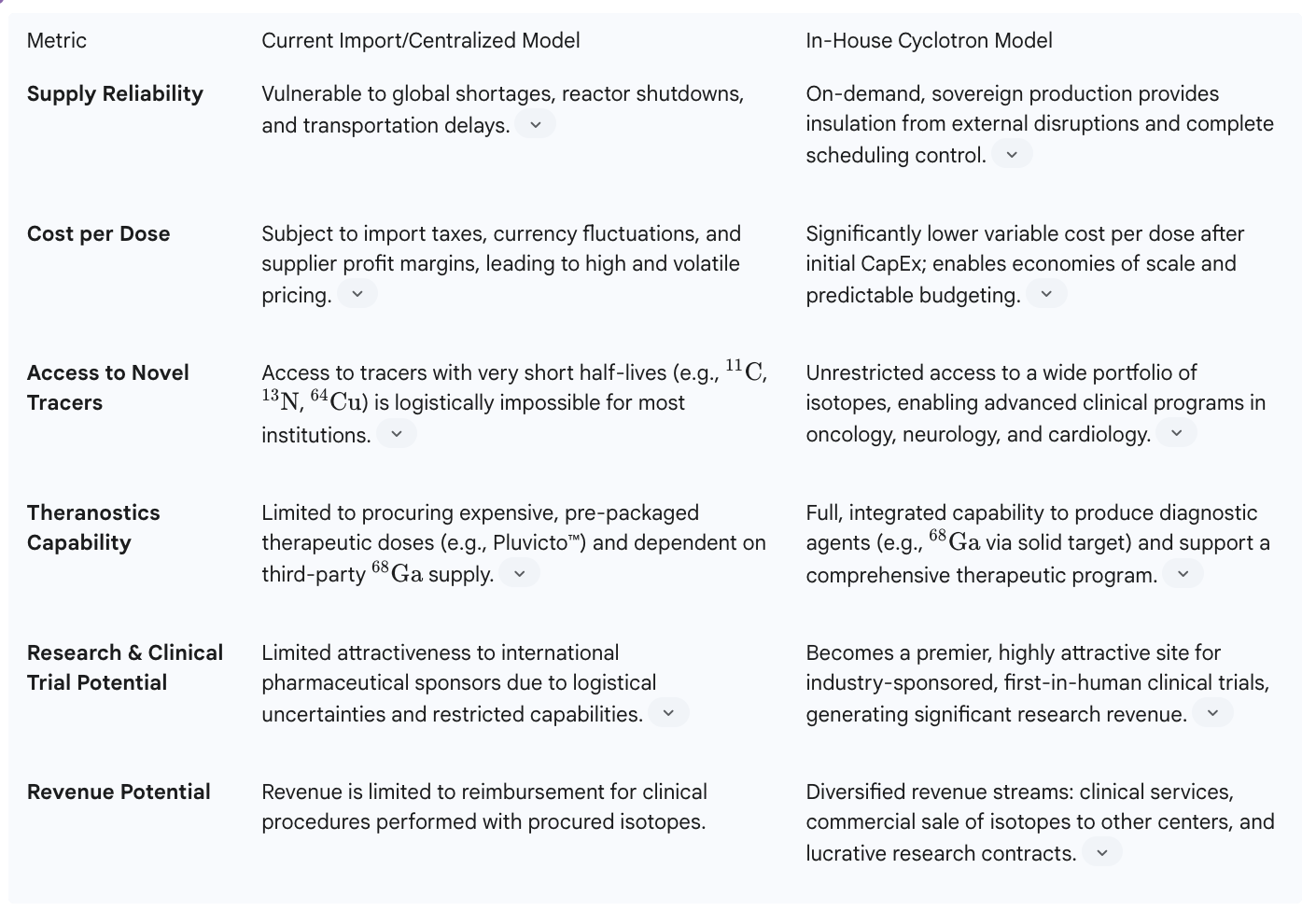
For a leading healthcare institution, the decision to invest in an in-house cyclotron and integrated radiopharmacy is not merely an operational upgrade; it is a fundamental strategic move toward vertical integration. This move transforms the hospital from a passive consumer at the end of a fragile supply chain into a sovereign producer that controls its own destiny. This shift provides the foundation for achieving market dominance through operational superiority, expanded clinical and research capabilities, and the creation of a new, powerful role as a regional infrastructure hub.
The most immediate and profound benefit of an in-house cyclotron is the achievement of operational sovereignty. By producing radiopharmaceuticals on-site, the hospital completely eliminates its reliance on external suppliers. This insulates the institution from the price shocks of currency fluctuations, the logistical nightmares of transportation delays, and the catastrophic disruptions caused by geopolitical events or distant reactor shutdowns. The radiopharmaceutical supply becomes a reliable, internal utility rather than a volatile external variable.
This control extends directly to patient care and operational efficiency. An on-site cyclotron provides complete command over the production schedule. This allows the nuclear medicine department to perfectly align isotope production with the demands of the PET/CT scanner schedule, maximizing patient throughput and asset utilization. It provides the flexibility to accommodate urgent or add-on cases without being constrained by a supplier's rigid delivery timetable. Furthermore, the ability to run multiple production cycles consecutively ensures a consistent and reliable supply throughout the day, a critical factor for a high-volume cancer center. This level of reliability and flexibility becomes a core operational strength, enhancing the quality of patient care and creating a key point of differentiation that is highly valued by referring physicians and patients alike.
Vertical integration does more than just secure the supply of existing radiopharmaceuticals; it unlocks an entirely new arsenal of clinical and research tools that are inaccessible to dependent institutions. On-site production liberates the hospital from the logistical constraints of short half-lives, enabling the routine use of a much broader portfolio of PET isotopes beyond the standard 18F-FDG used for general oncology.
This expanded capability allows for the development of advanced, specialized clinical programs. For instance, the hospital can begin producing and using 13N-Ammonia for highly accurate myocardial perfusion imaging in cardiology, or 11C-PiB for cutting-edge research and diagnosis of Alzheimer's disease in neurology. In oncology, it opens the door to producing novel diagnostic agents and, crucially, the diagnostic components of theranostic pairs. Modern cyclotrons equipped with solid target technology can efficiently produce Gallium-68 (
68Ga), the key diagnostic isotope for PSMA and DOTATATE imaging, as well as emerging radiometals like Copper-64 (64Cu) that are vital for the next generation of theranostics.
This capability is a game-changer for research. It transforms the institution into a hub for medical innovation. The ability to produce novel, short-lived radiotracers on-demand makes the hospital an exceptionally attractive partner for pharmaceutical and biotechnology companies. It becomes a prime location for pioneering new diagnostic procedures and participating in—or even leading—prestigious, first-in-human clinical trials for the next wave of radiopharmaceuticals. This is a level of research engagement that is simply impossible for competitors who lack this fundamental infrastructure, creating a deep and lasting competitive moat.
A modern medical cyclotron is an engine of immense productivity, with a capacity that often far exceeds the daily needs of a single institution. This excess capacity is not a liability; it is a significant commercial asset. By establishing a GMP-compliant radiopharmacy alongside the cyclotron, the hospital can commercialize this surplus production. It can begin selling unit doses of
18F-FDG and other radiopharmaceuticals to the network of surrounding hospitals, clinics, and imaging centers that lack their own production capabilities.
This strategy creates a new, high-margin business line that diversifies the hospital's revenue base. It turns a strategic clinical asset into a regional utility and a robust profit center. More importantly, it fundamentally reshapes the institution's role within the regional healthcare ecosystem. The hospital is no longer just another provider of care; it becomes a critical piece of infrastructure, the central hub upon which other facilities depend for their own advanced imaging services. This strategic repositioning from a consumer to a producer and distributor is a profound shift in market power. It elevates the institution above the competitive fray, turning former competitors for a limited supply of isotopes into a captive customer base. This "hub-and-spoke" model generates direct revenue while simultaneously building a network of dependent relationships, cementing the hospital's status as the undisputed market leader in advanced molecular imaging and oncology.
The strategic and clinical arguments for establishing an in-house theranostics program are compelling, but for C-suite executives and hospital boards, the decision ultimately hinges on financial viability. A common misconception views the cyclotron as an insurmountable expense. However, a detailed financial analysis reveals that a vertically integrated program, when structured as a multi-pillar business enterprise, transitions from a perceived cost center into a powerful and sustainable profit engine. This section deconstructs the investment and outlines a clear, data-driven pathway to a strong return on investment (ROI).
A transparent assessment of the required investment is the first step in any credible financial plan. The costs can be broken down into two main categories:
The key to unlocking profitability is to move beyond a single-minded focus on internal clinical use and architect a business model built on multiple, synergistic revenue streams. This diversification not only accelerates the return on investment but also creates a more resilient and financially robust program.
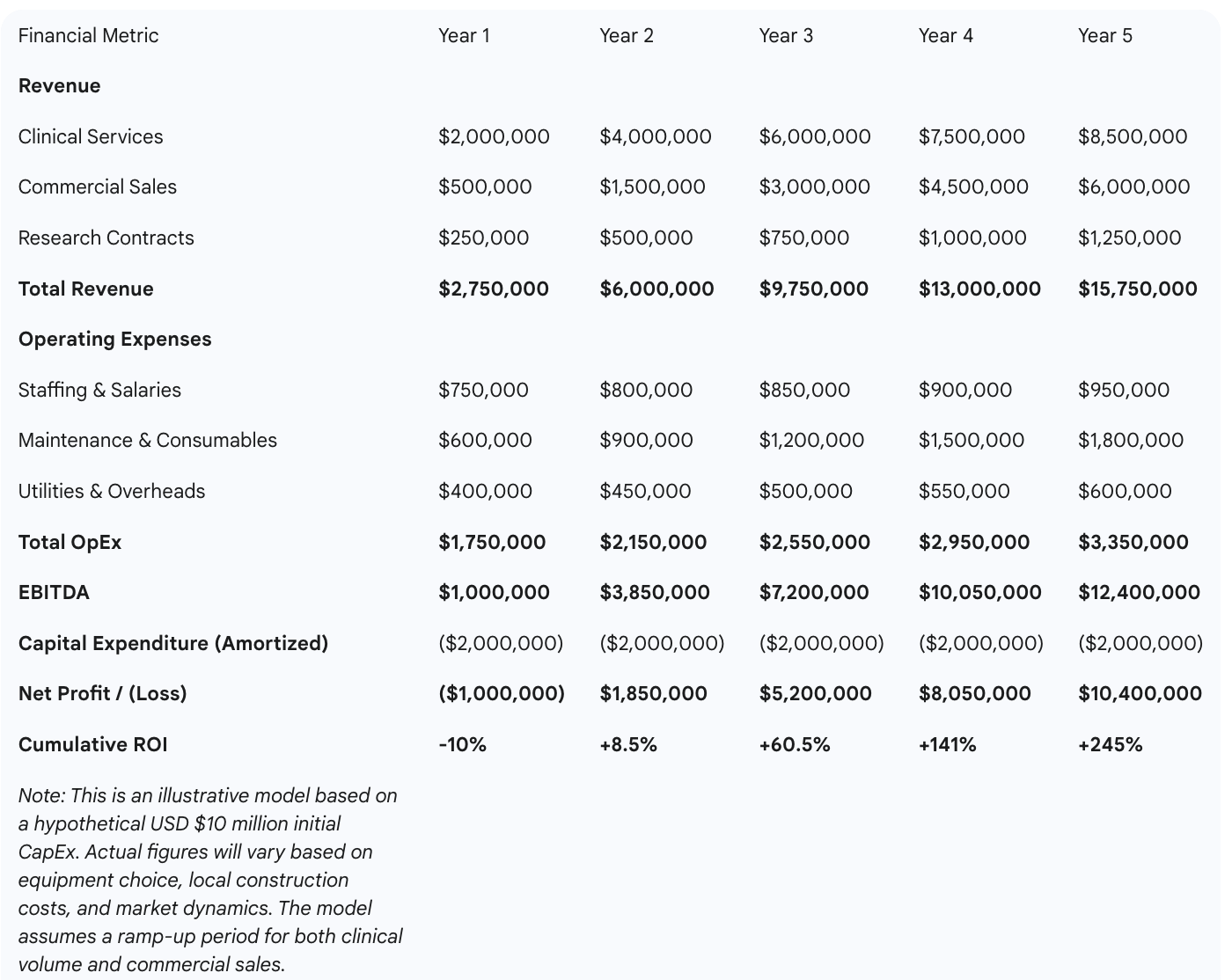
A comprehensive ROI model must integrate all three revenue pillars. Financial projections show that while the initial capital outlay is significant, the diversified revenue model creates a clear path to profitability. Break-even analysis is highly sensitive to the volume of clinical procedures and, crucially, the success in building the commercial distribution business. Conservative models, based on achieving moderate market penetration in regional distribution and securing a steady pipeline of clinical trials, suggest a capital payback period of approximately five to seven years.
The financial risk of this investment can be significantly mitigated through a proactive, phased business development strategy. The period of construction and commissioning of a cyclotron facility, which can take 12 to 18 months, should not be a period of financial inactivity. Instead, it presents a critical window to de-risk the investment. During this time, a dedicated business development team can be actively negotiating and signing letters of intent and formal supply agreements with regional clinics and hospitals. Simultaneously, the clinical research office can leverage the promise of the new, state-of-the-art facility to engage with pharmaceutical sponsors and secure participation in their upcoming Phase II and Phase III trial pipelines. This proactive approach ensures that on the very first day of operation, the cyclotron is serving not only the hospital's internal needs but also a pre-secured external customer base and contracted research partners. This strategy ensures that all major revenue streams are active from the outset, dramatically shortening the time to break-even and accelerating the journey to sustained profitability.
The decision to undertake a project of this magnitude transcends mere financial analysis; it is a question of vision, legacy, and leadership. For Brazil's premier healthcare institutions, particularly one with the history and ambition of Hospital Israelita Albert Einstein, this investment represents a defining moment—an opportunity not just to follow global trends, but to set the standard for advanced medical care in Latin America.
An investment in a vertically integrated theranostics program is not a deviation from the core mission of an institution like Albert Einstein; it is a powerful affirmation of it. It aligns perfectly with a long-established reputation for pioneering medical excellence, embracing innovation, and providing the highest quality of patient care. This is the logical and necessary next step in that legacy.
The hospital already possesses many of the critical components for success. It has a robust and respected nuclear medicine department, a world-class clinical research center with a history of significant contributions, and established post-graduate teaching programs in relevant fields like radiopharmacy and medical physics. These are invaluable assets. However, without the foundational infrastructure of an in-house cyclotron, their full potential remains unrealized. The cyclotron is the missing engine required to power these existing assets into a new era of discovery and clinical application. This investment would directly synergize with other major strategic initiatives, such as the recently announced BRL 380 million investment in a new Center for Care and Advanced Therapies in Oncology and Hematology, by providing that center with the next-generation diagnostic and therapeutic tools it will need to fulfill its promise.
An in-house cyclotron and radiopharmacy program does more than treat patients; it cultivates talent. It creates an unparalleled, hands-on training environment for the next generation of medical leaders. Residents, fellows, and post-graduate students in nuclear medicine, medical physics, oncology, and radiopharmacy will be drawn to an institution that offers direct experience with the entire lifecycle of radiopharmaceuticals, from production to clinical application. This will create a pipeline of top-tier talent, ensuring the institution's intellectual and clinical leadership for years to come.
By pioneering this integrated model in Brazil, the institution will be uniquely positioned to collaborate with key national bodies. It can work alongside the National Health Surveillance Agency (ANVISA) and the National Nuclear Energy Commission (CNEN) to help shape the future of theranostics regulation, GMP standards, and best practices in the country. It can partner with the Brazilian Society of Nuclear Medicine (SBMN) to develop national guidelines and training programs, solidifying its role not just as a provider of care, but as a national thought leader and standard-bearer for the entire specialty.
The opportunity to establish market dominance is immediate, but the window is finite. The radioligand therapy (RLT) sector has transitioned from a niche area of oncology to a strategic battleground for major pharmaceutical companies, validated by the blockbuster success of drugs like Pluvicto® and Lutathera®. This has triggered a wave of multi-billion-dollar acquisitions by companies like Eli Lilly, Bristol Myers Squibb, and AstraZeneca, with over $7.9 billion committed to acquire RLT assets. The global market is projected to grow from $6.8 billion in 2024 to over $14 billion by 2032.
However, this explosive growth is colliding with a fragile and underdeveloped clinical infrastructure worldwide. The primary constraint on RLT development is now a severe shortage of trial-ready clinical sites, specialized workforce, and advanced imaging capacity. With over 68 companies developing RLTs and more than 120 active clinical trials, sponsors are facing a "site activation nightmare," competing for a dangerously small number of qualified research centers.
This global bottleneck represents Brazil's single greatest opportunity to become the undisputed leader in advanced cancer care for Latin America. While other nations struggle with fragmented services and regulatory delays, a Brazilian institution that builds a purpose-built, integrated theranostics center can position itself as the premier destination for high-value clinical research in the region. This is not a theoretical advantage. A purpose-built RLT clinical trial site is a highly attractive, non-competitive partner for pharmaceutical sponsors, designed to alleviate the capacity and capital constraints faced by traditional health systems. By establishing this capability, the institution would not just be serving Brazilian patients; it would be attracting millions in foreign investment from global pharma, creating a competitive advantage protected by a high barrier to entry and cementing its role as the innovation hub for the entire continent.
Furthermore, this strategic investment can be powerfully positioned as a vehicle for public-private partnership (PPP), enhancing the institution's social responsibility profile. The public health system (SUS) faces significant challenges in providing access to advanced diagnostics and cancer care. A hospital with its own cyclotron possesses a unique, high-value asset that can be leveraged to address these national needs. It can establish formal partnerships to provide PET/CT scans and theranostic treatments to SUS patients at negotiated rates, helping to alleviate public system backlogs and improve healthcare equity. This not only opens an additional, stable revenue stream but also generates immense public goodwill, strengthens the hospital's philanthropic mission, and positions it as a collaborative leader working to solve Brazil's most pressing healthcare challenges.
The evidence presented in this report leads to an unequivocal conclusion: the shift to theranostics is not a fleeting trend but an irreversible and accelerating evolution in the standard of cancer care. The core question for Brazil's healthcare leaders is no longer if this technology will become central to oncology, but rather who will have the foresight and courage to lead this transformation.
The strategic choice is stark. An institution can choose to remain a passive consumer, subject to the whims of a fragile, restrictive, and increasingly inadequate global supply chain. This path leads to operational constraints, limited clinical innovation, and a gradual erosion of competitive advantage as the world of medicine moves forward. Alternatively, it can choose to become a sovereign producer—an institution that controls its own technological destiny, defines the regional market, and offers a level of personalized, cutting-edge care that is simply unattainable by its competitors. This is a chance to participate directly in a multi-billion-dollar global industry, not just observe it from the sidelines.
The time for deliberation is ending, and the time for decisive action is here. The path to leadership requires investment in the foundational infrastructure of 21st-century medicine. We strongly urge the executive leadership and board of directors to commission a formal, detailed feasibility study and financial analysis for the development of an in-house medical cyclotron and integrated theranostics center. This is the essential first step toward making a legacy-defining investment that will secure the institution's leadership position, attract a new generation of world-class talent, and fundamentally change the face of cancer care for patients across Brazil.
1. What exactly is theranostics?Theranostics is an advanced approach in medicine that combines "therapy" and "diagnostics" into a single process. It uses specialized radioactive drugs, called radiopharmaceuticals, that have two key parts: a targeting molecule that binds to specific markers on cancer cells, and a radioactive isotope. First, a low-energy version is used with PET or SPECT scans to precisely locate and visualize tumors. If the diagnostic scan confirms the target is present, a high-energy version of the same targeting molecule is used to deliver radiation directly to the cancer cells, destroying them while minimizing damage to surrounding healthy tissue.
2. Why is an in-house cyclotron essential for a top-tier theranostics program in Brazil?An in-house cyclotron is essential because the most advanced radiopharmaceuticals used for PET imaging and theranostics have extremely short half-lives—often less than two hours. This makes it logistically impossible to import them or transport them over long distances. Brazil's current reliance on a few production centers, mainly in the Southeast, creates a major bottleneck and restricts access for most of the country. An on-site cyclotron eliminates this dependency, ensuring a reliable, on-demand supply of these critical agents. This in turn allows for flexible patient scheduling, expanded clinical capabilities, and insulation from global supply chain disruptions. Critically, it is also a prerequisite for attracting lucrative international clinical trials, as the global industry is facing a severe shortage of trial-ready sites with this infrastructure.
3. What is the estimated financial investment for establishing a cyclotron facility?The investment is significant and can be broken into two main categories. Capital expenditure (CapEx) includes the cyclotron itself (USD $2.5–$6.6 million) and the necessary infrastructure, such as a shielded bunker and a GMP-compliant radiopharmacy, which can often double the total project budget. Operational expenditure (OpEx) includes recurring costs for specialized staff, maintenance contracts, raw materials, and utilities, which can amount to USD $1.5–$2 million annually.
4. How does such a significant investment generate a positive return (ROI)?A positive ROI is achieved by structuring the program as a multi-pillar business, not just a cost center for the hospital. The key revenue streams include:
5. What are the main regulatory steps involved?Establishing and operating a radiopharmacy and theranostics center in Brazil requires adherence to strict regulations from several bodies. The National Health Surveillance Agency (ANVISA) requires all manufacturing facilities to comply with Good Manufacturing Practices (GMP), as detailed in resolutions like RDC 658/2022. Additionally, the National Nuclear Energy Commission (CNEN) regulates all activities involving radioactive materials, including licensing for personnel and facilities to ensure radiation safety and protection. A successful program requires close collaboration with these agencies to ensure full compliance.
6. Beyond treating patients, what other benefits does this program bring to the hospital and the community?A state-of-the-art theranostics center acts as a hub for innovation and excellence. It attracts top-tier medical talent—physicians, physicists, and researchers—who want to work at the forefront of medicine, addressing a global talent crisis in this specialized field. It becomes an unparalleled training ground for the next generation of specialists, creating a sustainable pipeline of expertise. The institution also becomes a national standard-bearer, helping to shape best practices and regulations. Furthermore, it opens opportunities for Public-Private Partnerships (PPPs) to provide advanced care to patients in the public system (SUS), enhancing healthcare equity and fulfilling a crucial social mission.
https://synapselatam.com/https://www.iaea.org/bulletin/brazils-experiencehttps://www.researchgate.net/publication/388432448_Nuclear_Medicine_in_Brazilian_Health_Systemhttps://f1000research.com/articles/13-283https://jnm.snmjournals.org/content/jnumed/early/2022/04/21/jnumed.122.264321.full.pdfhttps://www.marketsandmarkets.com/Market-Reports/theranostics-market-111696976.htmlhttps://www.mordorintelligence.com/industry-reports/medical-cyclotron-markethttps://www.emergenresearch.com/industry-report/the-medical-cyclotron-markethttps://agencia.fapesp.br/radiopharmaceutical-synthesized-in-brazil-improves-quality-of-life-for-prostate-cancer-patients/53457https://pmc.ncbi.nlm.nih.gov/articles/PMC7367151/https://sbmn.org.br/https://www.gov.br/anvisa/pt-br/english/regulation-of-companies