


The Dominican Republic has emerged as a premier destination for medical device clinical trials, offering a unique blend of advantages that appeal to sponsors and researchers alike. With a regulatory framework that aligns with international standards and significantly faster approval times compared to North America and Europe, the country is positioned to facilitate efficient trial execution. The cost-effectiveness of conducting trials here—approximately 40% lower than in more developed markets—combined with a diverse patient population, enhances the feasibility of obtaining generalizable results.
As the clinical research landscape evolves, understanding the key considerations for selecting the right Contract Research Organization (CRO) and recognizing the future trends shaping this sector will be essential for stakeholders aiming to leverage the Dominican Republic's capabilities in advancing medical technology and improving patient outcomes.
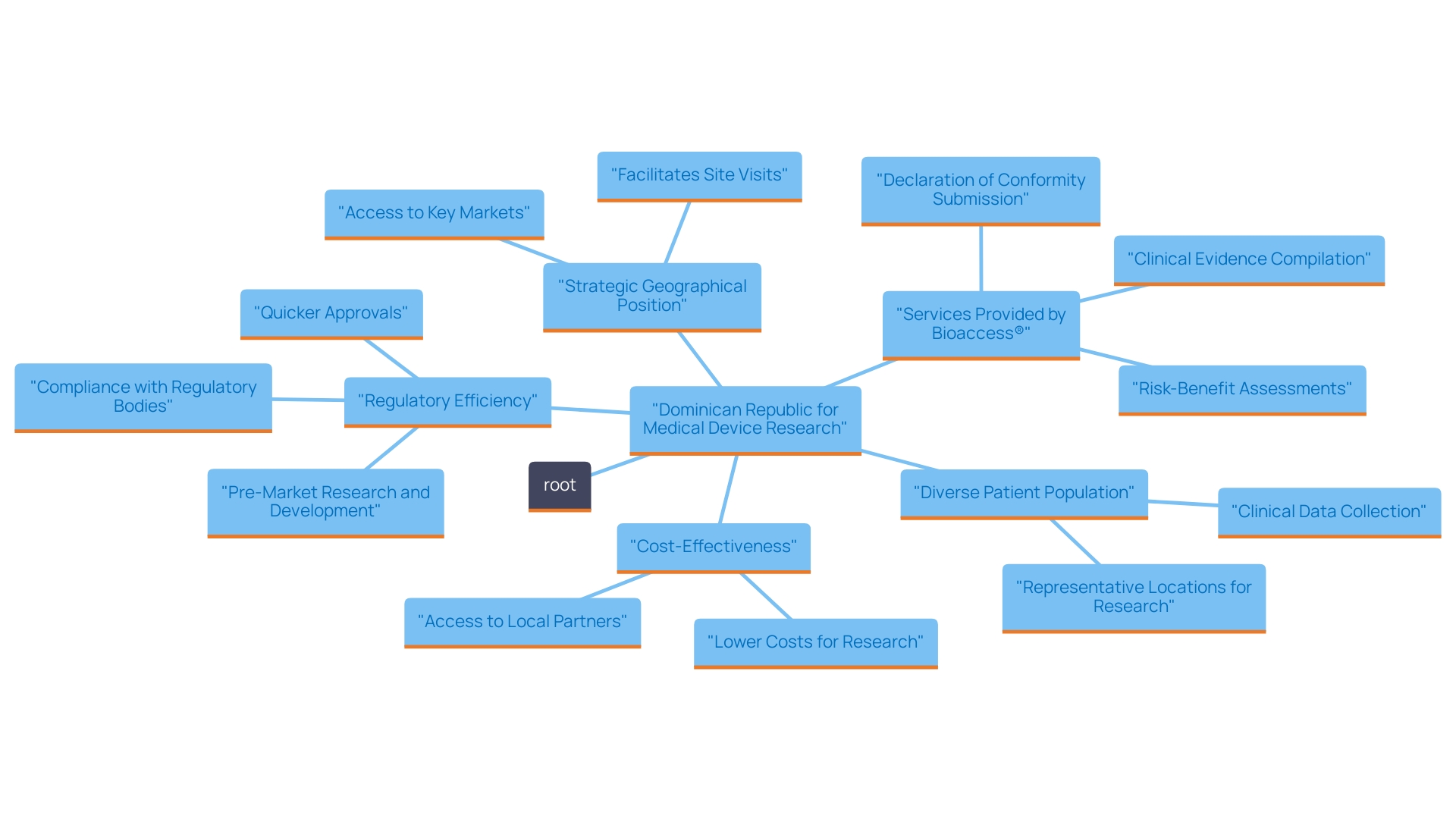
The Dominican Republic has emerged as an appealing location for medical device research studies due to several key advantages.
Firstly, the country boasts a well-established regulatory framework that aligns with international standards, facilitating smoother approvals and compliance processes. For example, research study approval durations in the Dominican Republic can be up to 30% quicker than in North America and Europe, greatly shortening time-to-market for innovative products.
Secondly, the expense of carrying out medical studies in the Dominican Republic is around 40% less than in North America, offering economical options for sponsors.
The availability of a diverse patient population, with over 10 million residents representing various demographics, further enriches the research landscape, enabling the recruitment of participants essential for generalizable results.
Moreover, the country’s strategic geographical position permits easy access to both North and South American markets, enhancing logistical efficiency for management.
Furthermore, bioaccess® provides extensive research study management services, including:
These combined factors create an appealing setting for conducting high-quality clinical studies of healthcare equipment, ultimately leading to enhanced patient results, expedited time-to-market for innovative products, and substantial positive effects on local economies through job creation and economic development.
CRO Solutions DR: Recognized for its strong focus on compliance and quality, CRO Solutions DR offers a comprehensive range of services customized for healthcare products. Their skilled team guarantees effective execution within regulatory guidelines, establishing them as a trustworthy partner for sponsors.
Clinica de Investigación: This CRO boasts extensive experience in medical device studies, particularly in the cardiovascular and orthopedics sectors. They excel in project management and have a proven track record of successful execution.
Dominican Clinical Research: With an emphasis on patient-focused studies, Dominican Clinical Research effectively recruits diverse populations, ensuring that research yields valid and applicable results. Their cost-effective solutions and extensive healthcare network make them a preferred choice for many sponsors.
MedTrials: Specializing in healthcare equipment studies, MedTrials provides competitive pricing along with a strong dedication to quality. Their expertise in navigating the regulatory landscape provides stakeholders with peace of mind.
Apex Clinical Research: Apex distinguishes itself through a personalized approach to project management, ensuring that each trial is tailored to the specific needs of the client. Their innovative approaches and knowledge in healthcare instruments make them a valuable research collaborator.
Global Research Solutions: Acknowledged for operational efficiency and cost management, Global Research Solutions supports a range of healthcare studies while prioritizing compliance and patient safety.
Innova Research: With a strong emphasis on technological integration, Innova Research offers unique solutions that streamline processes and enhance data integrity. Their competitive rates and knowledge in healthcare assessments position them as an ideal option for sponsors.
bioaccess®: With more than 20 years of expertise in Medtech, bioaccess® focuses on expedited healthcare product research services throughout Latin America. They offer extensive study management services, including feasibility assessments, site selection, compliance evaluations, setup, import permits, project oversight, and reporting. As a vetted CRO and consulting partner for U.S. healthcare companies in Colombia, bioaccess® not only ensures efficient execution of studies but also drives significant local economic impacts, such as job creation, healthcare improvement, and international collaboration.
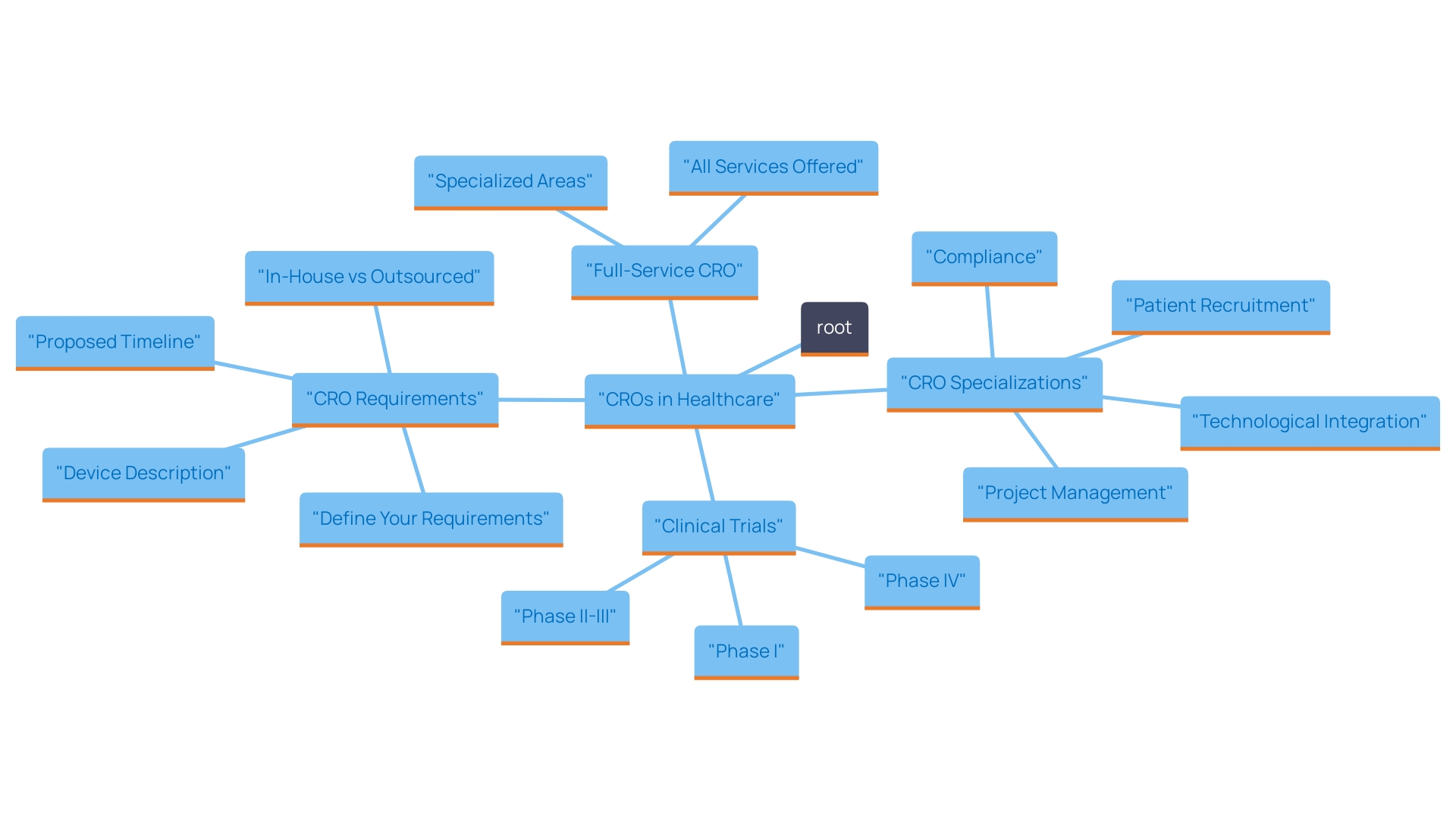
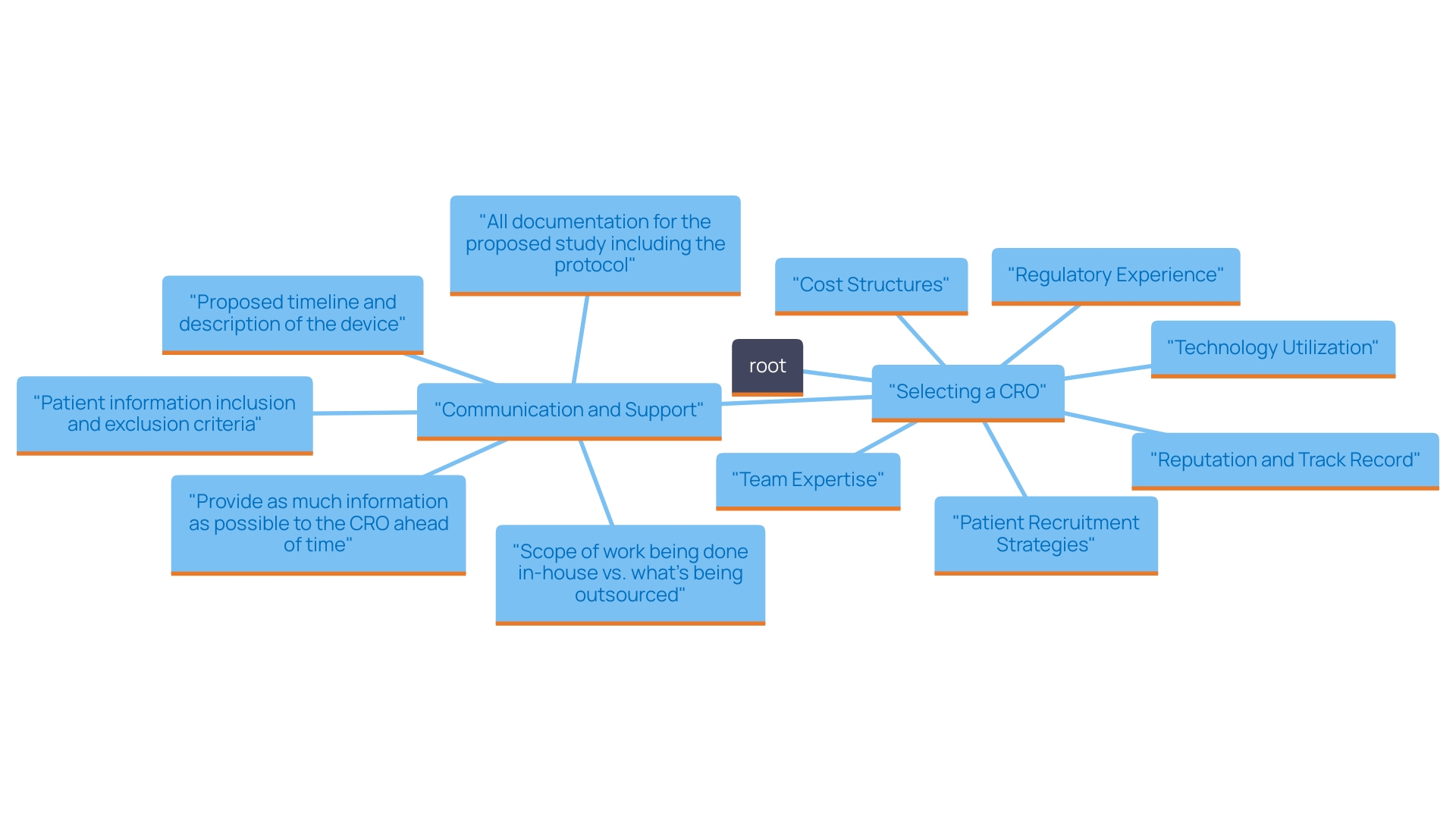
When selecting a CRO for medical device clinical trials in the Dominican Republic, consider the following key factors:
In summary, choosing the appropriate CRO is vital for the success of your research studies. By thoughtfully evaluating these factors, you can guarantee a seamless and efficient process that meets all regulatory requirements and fulfills your research objectives.
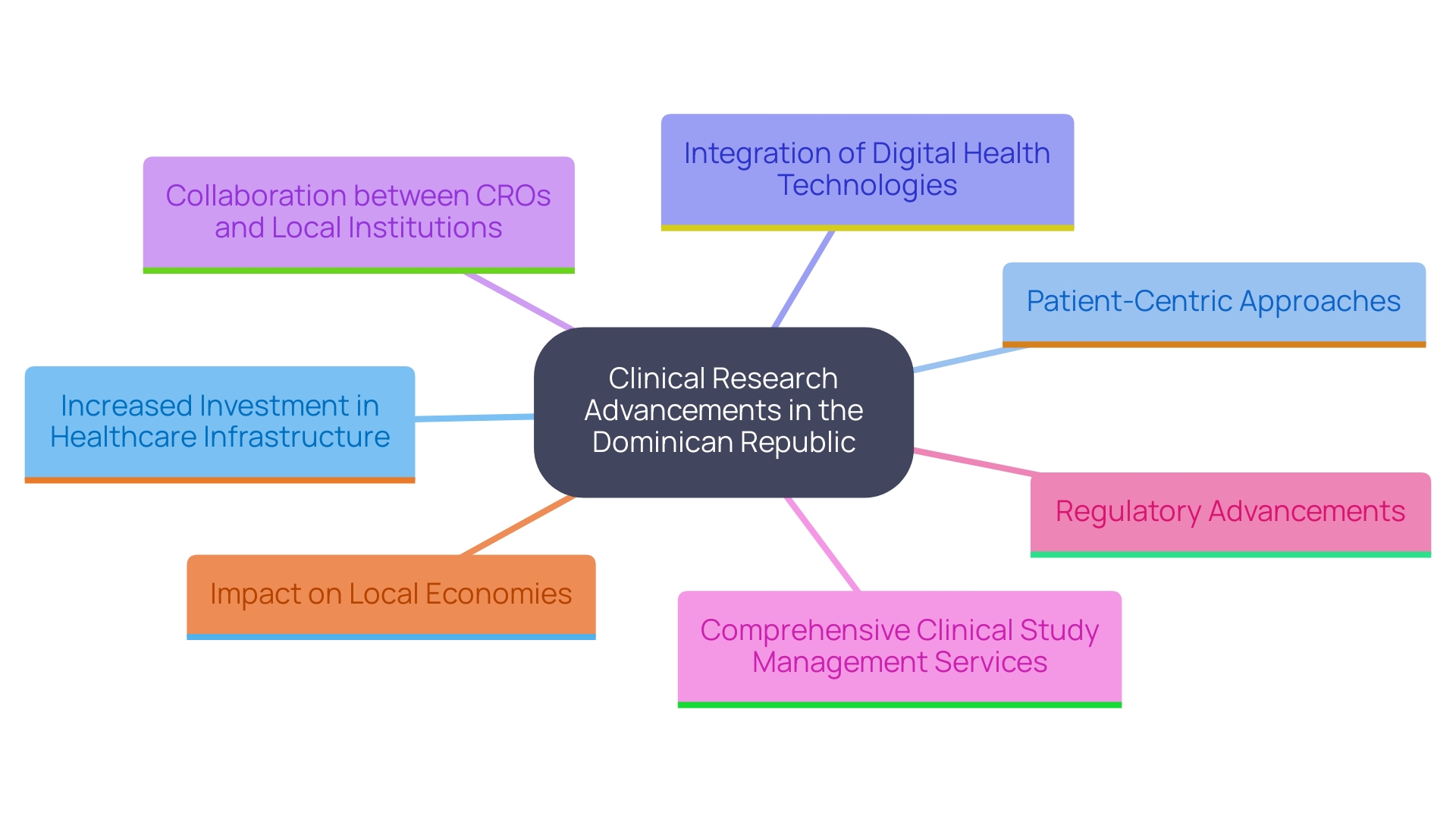
The clinical research landscape in the Dominican Republic is poised for significant advancements driven by several key trends:
The Dominican Republic stands out as an exceptional location for conducting medical device clinical trials, thanks to its favorable regulatory environment, cost advantages, and diverse patient population. The combination of faster approval times and lower operational costs—approximately 40% less than in North America—positions the country as a strategic choice for sponsors seeking efficient trial execution. Furthermore, the presence of numerous capable Contract Research Organizations (CROs) enhances the feasibility and effectiveness of clinical studies, ensuring adherence to high-quality standards and regulatory compliance.
As the clinical research landscape evolves, key considerations in selecting a CRO become paramount. Evaluating factors such as regulatory experience, team expertise, and patient recruitment strategies can significantly influence the success of clinical trials. Moreover, the integration of digital health technologies and a focus on patient-centric approaches are shaping the future of clinical research in the Dominican Republic, promising improved outcomes and more relevant study designs.
In conclusion, the Dominican Republic offers a unique blend of advantages for medical device clinical trials. By leveraging its strengths—efficient regulatory processes, cost-effectiveness, and a commitment to high-quality research—stakeholders can capitalize on the opportunities presented in this vibrant market. As the healthcare landscape continues to advance, embracing these trends will be essential for driving innovation and enhancing patient outcomes in the region.
Why is the Dominican Republic considered an appealing location for medical device research studies?
The Dominican Republic offers a well-established regulatory framework that aligns with international standards, allowing for quicker approvals, reduced costs (about 40% less than in North America), a diverse patient population, and strategic geographical access to North and South American markets.
How much faster can research study approvals be in the Dominican Republic compared to North America and Europe?
Research study approval durations in the Dominican Republic can be up to 30% quicker than in North America and Europe.
What services does bioaccess® provide for research study management in the Dominican Republic?
bioaccess® offers services including feasibility assessments, investigator selection, compliance evaluations, study setup, import permits, and project management.
What are some Clinical Research Organizations (CROs) operating in the Dominican Republic?
Some CROs include CRO Solutions DR, Clinica de Investigación, Dominican Clinical Research, MedTrials, Apex Clinical Research, Global Research Solutions, Innova Research, and bioaccess®.
What factors should be considered when selecting a CRO for medical device clinical trials in the Dominican Republic?
Key factors include regulatory experience, reputation and track record, cost structures, team expertise, patient recruitment strategies, technology utilization, and communication and support.
What trends are shaping the clinical research landscape in the Dominican Republic?
Key trends include increased investment in healthcare infrastructure, emphasis on patient-centric approaches, integration of digital health technologies, collaboration between CROs and local institutions, comprehensive clinical study management services, regulatory advancements, and positive impacts on local economies.
How do clinical trials impact local economies in the Dominican Republic?
Clinical trials contribute to local economies by promoting job creation, economic growth, healthcare improvement, and fostering international collaboration, with studies indicating a 15% increase in local employment opportunities in research-related fields.