


This article examines the premier job opportunities for senior Clinical Research Associates (CRAs) that can significantly propel one's career in clinical research. It underscores the escalating demand for CRAs in specialized sectors, particularly oncology and diabetes. The discussion emphasizes the critical importance of compliance and patient safety, alongside the potential for competitive salaries, which can range from $64,000 to $189,000 based on experience and specialization. This landscape not only highlights the career prospects but also the pivotal role CRAs play in advancing clinical research.
The clinical research landscape is rapidly evolving, presenting a wealth of opportunities for professionals eager to advance their careers. Senior Clinical Research Associate (CRA) positions are at the forefront of this transformation, particularly in specialized fields such as oncology and diabetes.
As the demand for skilled CRAs continues to grow, it becomes essential to understand the unique challenges and rewards of these roles for those aiming to excel in this competitive field.
What does it take to not only secure one of these coveted positions but also thrive in a dynamic environment where patient safety and regulatory compliance are paramount?
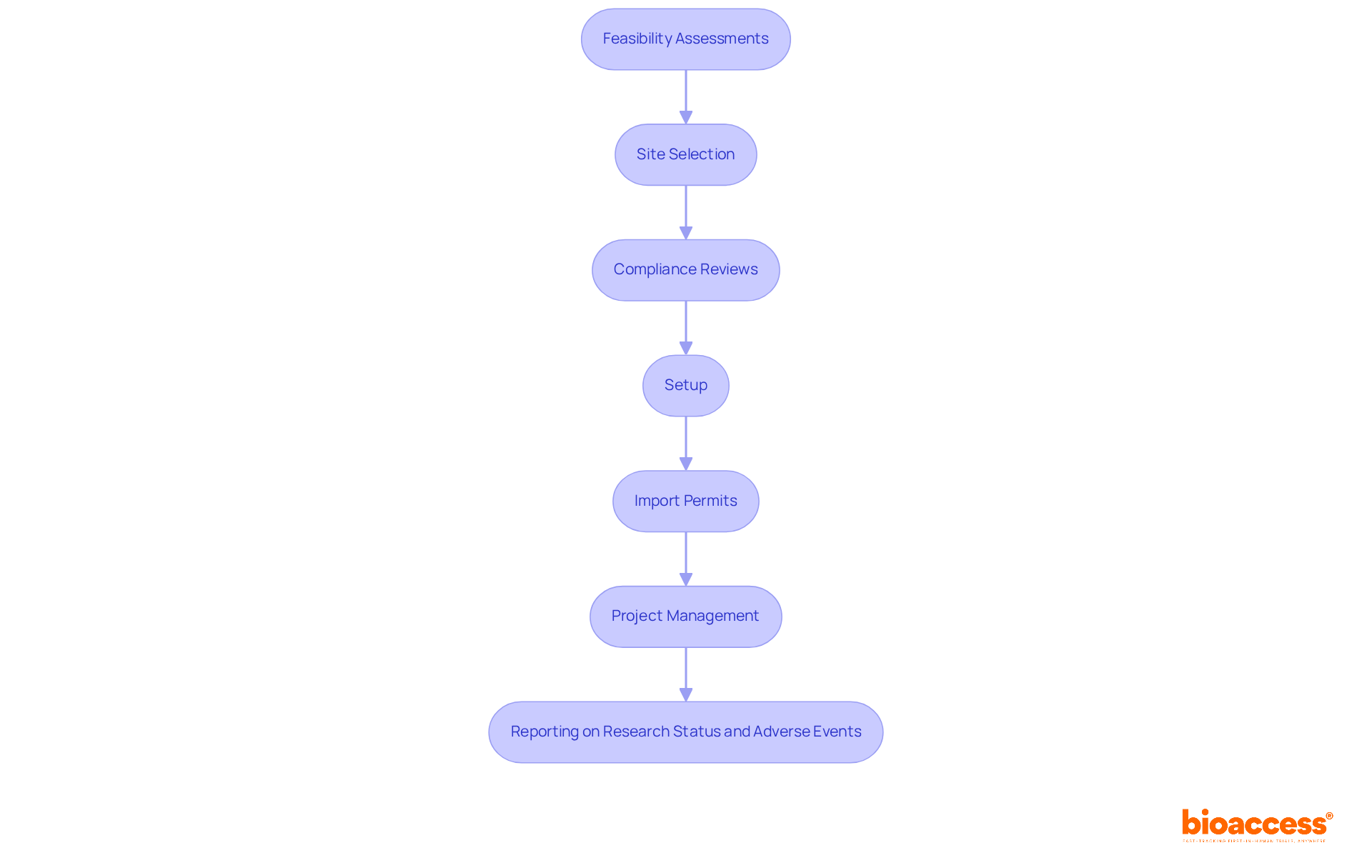
The Senior Clinical Research Associate (CRA) role at bioaccess® is pivotal in managing oncology investigations. The CRA is accountable for supervising the implementation of research while ensuring strict compliance with regulatory standards and protocols. This role demands an in-depth understanding of oncology-specific regulations and the ability to cultivate strong relationships with investigators and sponsors. CRAs play a vital role in monitoring patient safety and data integrity, which are essential for the advancement of cancer treatments.
At bioaccess®, our extensive management services for research encompass:
Recent trends in oncology clinical research indicate a growing emphasis on compliance, with investigations demonstrating that rigorous oversight is necessary to maintain data integrity and participant safety. For instance, oncology studies frequently highlight a high occurrence of serious adverse events (SAEs), underscoring the necessity for skilled clinical research associates to manage these challenges efficiently.
Successful case studies underscore the significance of senior CRA jobs for clinical research associates in oncology. A recent project illustrated that proactive management and compliance strategies led to improved patient recruitment and retention rates, ultimately enhancing study outcomes. Furthermore, senior CRA jobs involve conducting routine monitoring visits to review source documents and ensure informed consent is obtained, which is critical for safeguarding participant rights and data integrity. Additionally, benchmarking studies on site metrics from previous experiments have shown that research associates can enhance study performance, resulting in more efficient and effective research outcomes.
As the landscape of oncology research evolves in 2025, the demand for senior CRA jobs is increasing, as skilled CRAs who can ensure compliance and drive successful study execution become essential in the battle against cancer.
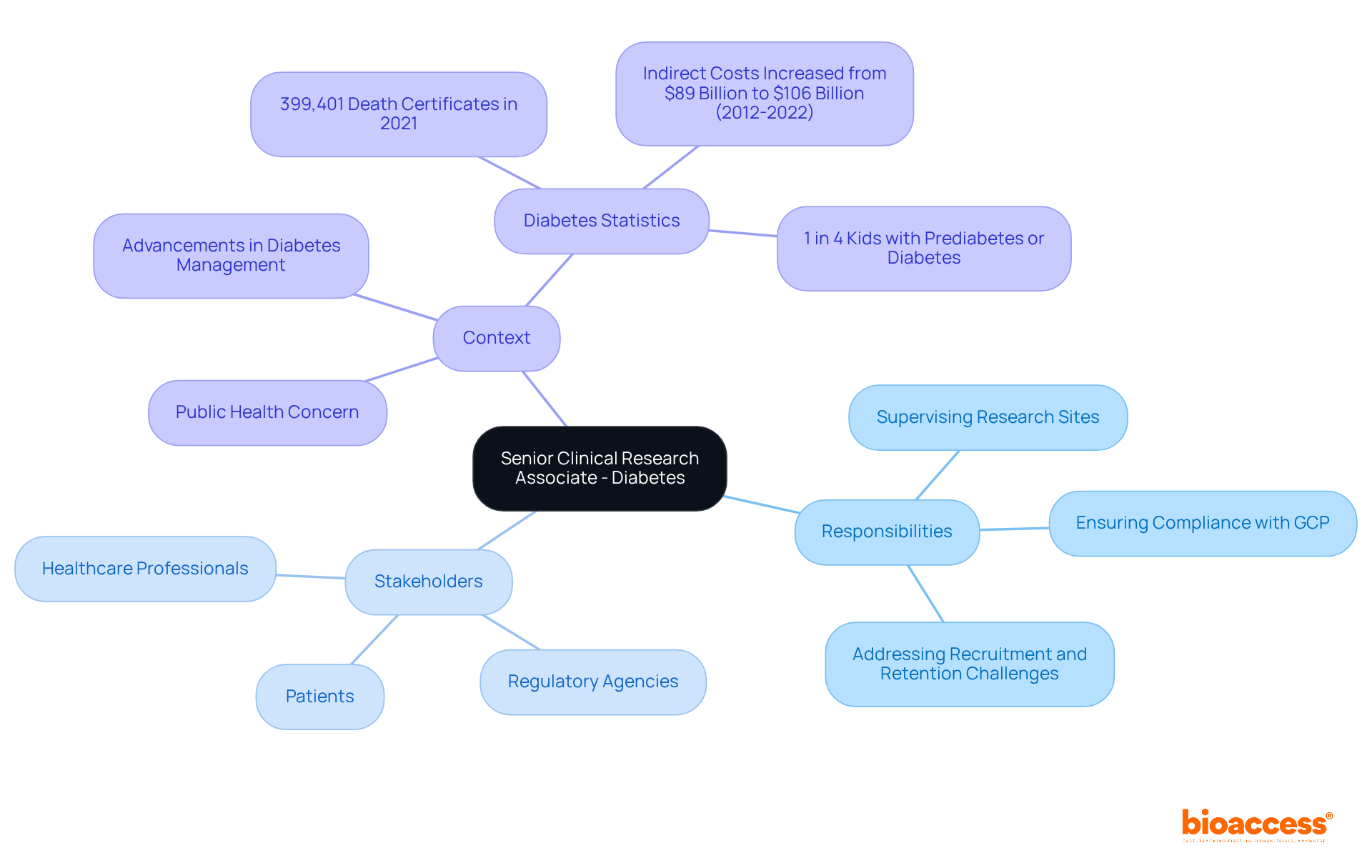
The senior CRA jobs for Diabetes on the West Coast will be remote positions, overseeing studies focused on enhancing diabetes management and treatment. This pivotal position necessitates effective coordination with a diverse array of stakeholders, including healthcare professionals and regulatory agencies, to ensure that studies are conducted efficiently and ethically.
Key responsibilities encompass:
Given that diabetes is a significant public health concern, the CRA will remain informed about the latest trends and advancements in diabetes management, thereby contributing to the overall success of research initiatives aimed at improving patient outcomes.
The Senior Clinical Research Associate (CRA) jobs for CNS/Ophthalmology in the Upper Midwest will play a crucial role in overseeing clinical studies that focus on neurological and eye health. This remote position for senior CRA jobs demands a comprehensive understanding of the unique challenges inherent in CNS and ophthalmology research, particularly concerning patient recruitment and retention.
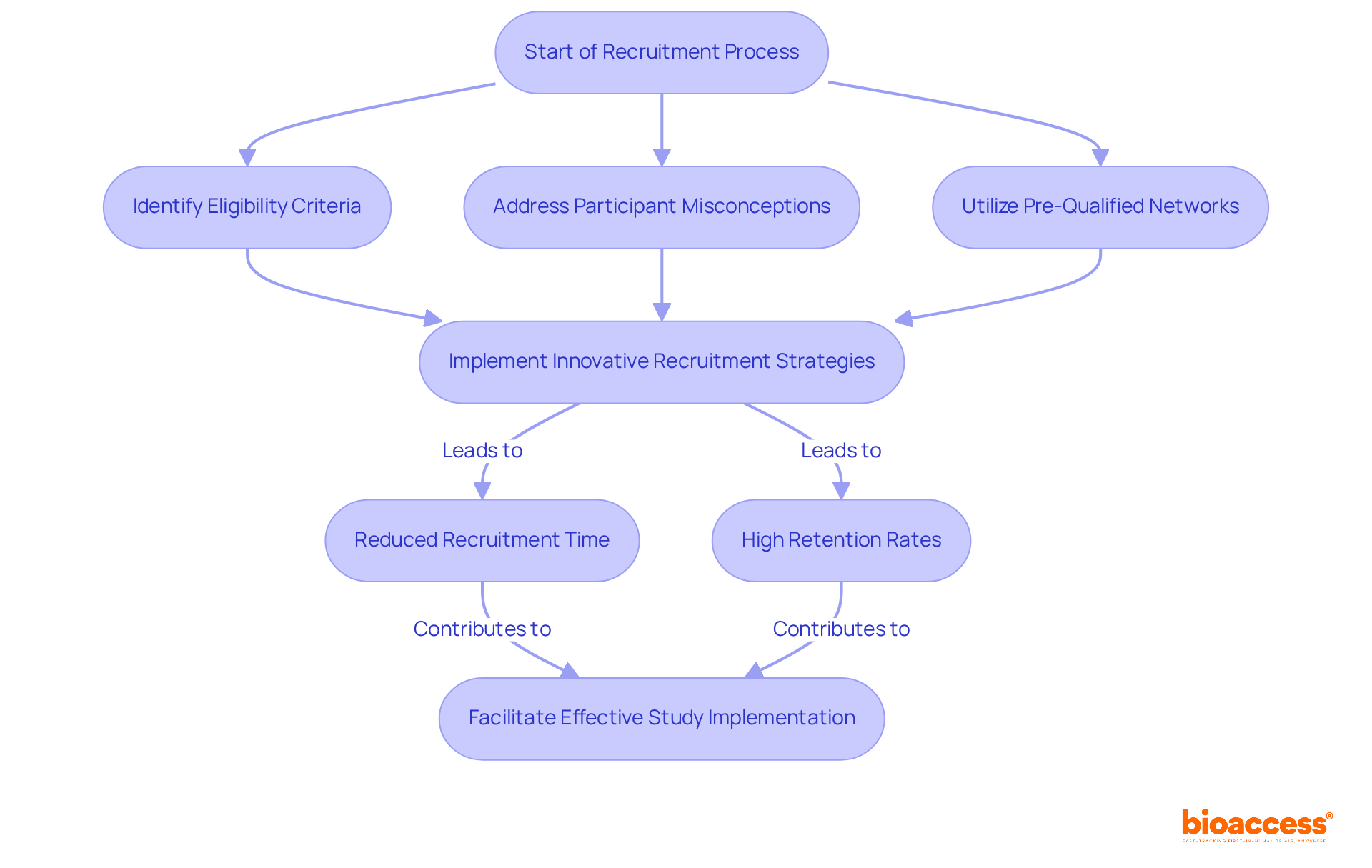
Effective recruitment strategies are essential, as studies in these areas often encounter significant hurdles, including stringent eligibility criteria and participant misconceptions about research risks. To address these challenges, bioaccess® offers accelerated patient recruitment and site activation services, leveraging pre-qualified networks of over 50 sites activated in less than 8 weeks and FDA/EMA/MDR-compliant datasets to streamline the process.
The CRA will ensure that all experimental activities adhere to regulatory standards while maintaining data integrity. By utilizing innovative recruitment methods and fostering strong connections with participants, the CRA will facilitate the effective implementation of studies, ultimately promoting the advancement of neurological research.
Notably, partnerships such as that of GlobalCare Clinical Trials with bioaccess™ have demonstrated the potential to reduce recruitment time by over 50% and achieve retention rates exceeding 95%, showcasing the effectiveness of these strategies.
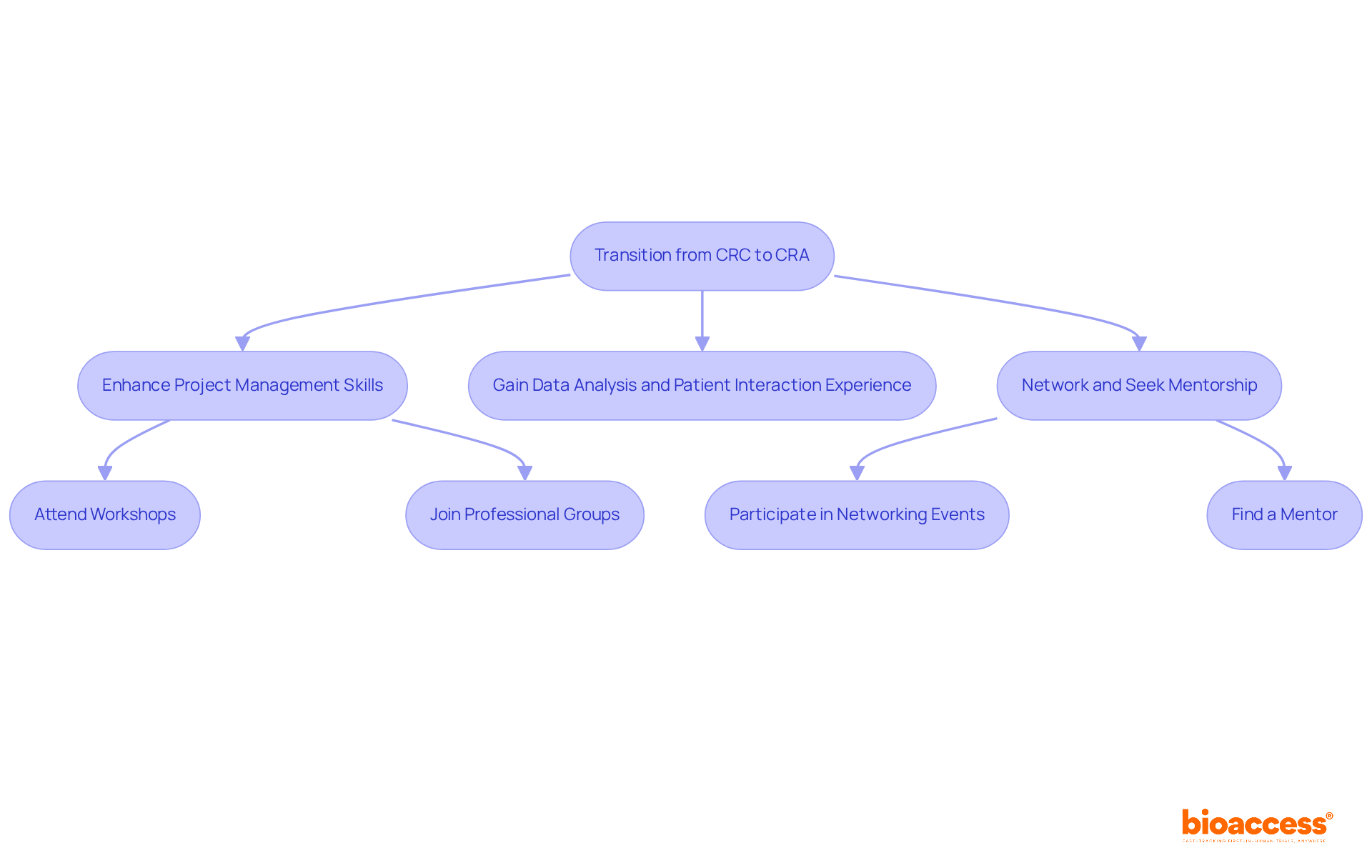
Transitioning from a Clinical Research Coordinator (CRC) to a home-based Clinical Research Associate (CRA) necessitates a comprehensive understanding of regulatory compliance and monitoring processes. It is crucial for CRCs to enhance their project management skills while also gaining experience in data analysis and patient interactions.
Networking with current CRAs and seeking mentorship can provide invaluable insights and support throughout this career shift. This strategic approach not only fosters professional growth but also prepares CRCs to navigate the complexities of their new role effectively.
The remote senior CRA jobs in diabetes research are pivotal for overseeing clinical studies aimed at enhancing diabetes therapies. Key responsibilities encompass:
This position is essential for upholding ethical research practices and prioritizing patient safety throughout the research lifecycle. In 2025, ethical considerations in diabetes research studies will undergo heightened scrutiny, with industry leaders emphasizing the necessity for transparency and informed consent. Effective site selection strategies are critical, as evidenced by case studies demonstrating that diverse patient populations significantly improve study outcomes. Statistics indicate that patient safety remains paramount, supported by rigorous monitoring protocols designed to mitigate risks associated with diabetes research. By fostering a culture of ethical responsibility, those in senior CRA jobs are instrumental in shaping the future of diabetes treatment and ensuring that clinical trials enhance patient care.
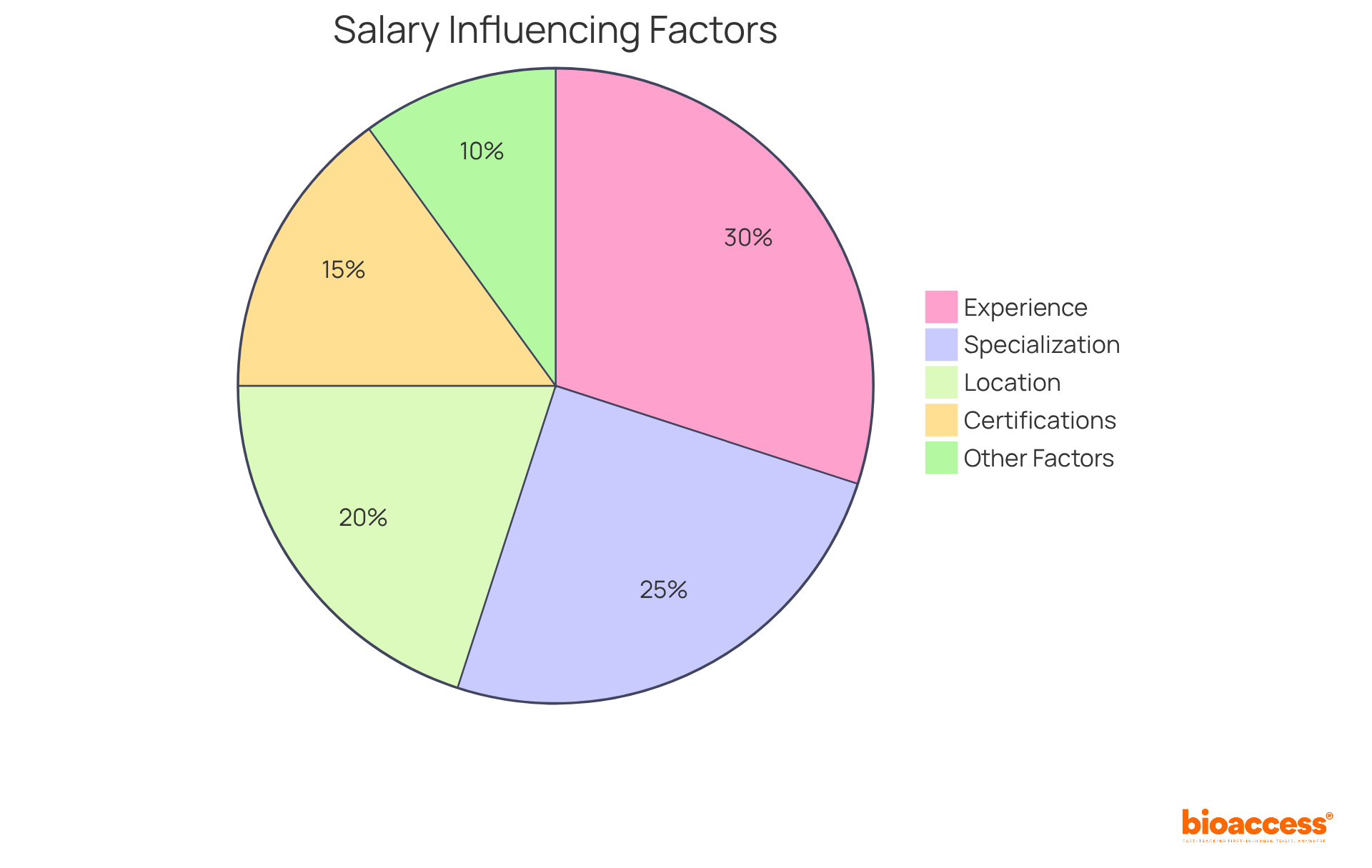
The salary range for senior CRA jobs is notably diverse, spanning from $64,000 to $189,000, influenced by several key factors. Experience plays an important role, with entry-level Clinical Research Associates earning approximately $70,000 to $85,000, while those with 3-5 years of experience can expect salaries between $95,000 and $115,000. Senior CRA jobs, particularly those that involve managing intricate multi-country trials, often surpass $130,000, with the highest documented salary reaching up to $145,000 each year.
Location greatly influences salary expectations, as clinical research associates in high-paying states such as California, New York, and Massachusetts earn 10-20% more than their rural peers. Additionally, specialization in high-demand therapeutic areas such as oncology, rare diseases, or gene therapies can lead to salary increases of 15-25% compared to general therapeutic areas. Clinical research associates involved in oncology, rare disease, or gene therapy studies receive 15-25% greater compensation compared to colleagues in general therapeutic fields.
The complexity of the clinical studies managed also influences compensation. Clinical research associates engaged in decentralized studies or those needing advanced digital abilities are increasingly compensated, with employers providing bonuses of 10-15% above median salaries for such expertise. Furthermore, certifications like ACRP-CP and CCRA can enhance earning potential by 10-20%, making them valuable assets for career advancement.
As the job market for clinical research associates remains competitive, with an estimated growth rate of 7% annually, professionals should consider these factors when evaluating job offers and negotiating salaries. Comprehending the dynamics of salary trends and the impact of specialization can enable clinical research associates to make informed career choices. Notably, the gender distribution among Senior Clinical Research Associates indicates that 80% are female, reflecting the demographic landscape of the profession. Furthermore, changing employers can lead to higher salary opportunities in senior CRA jobs, making it a strategic consideration for career advancement.
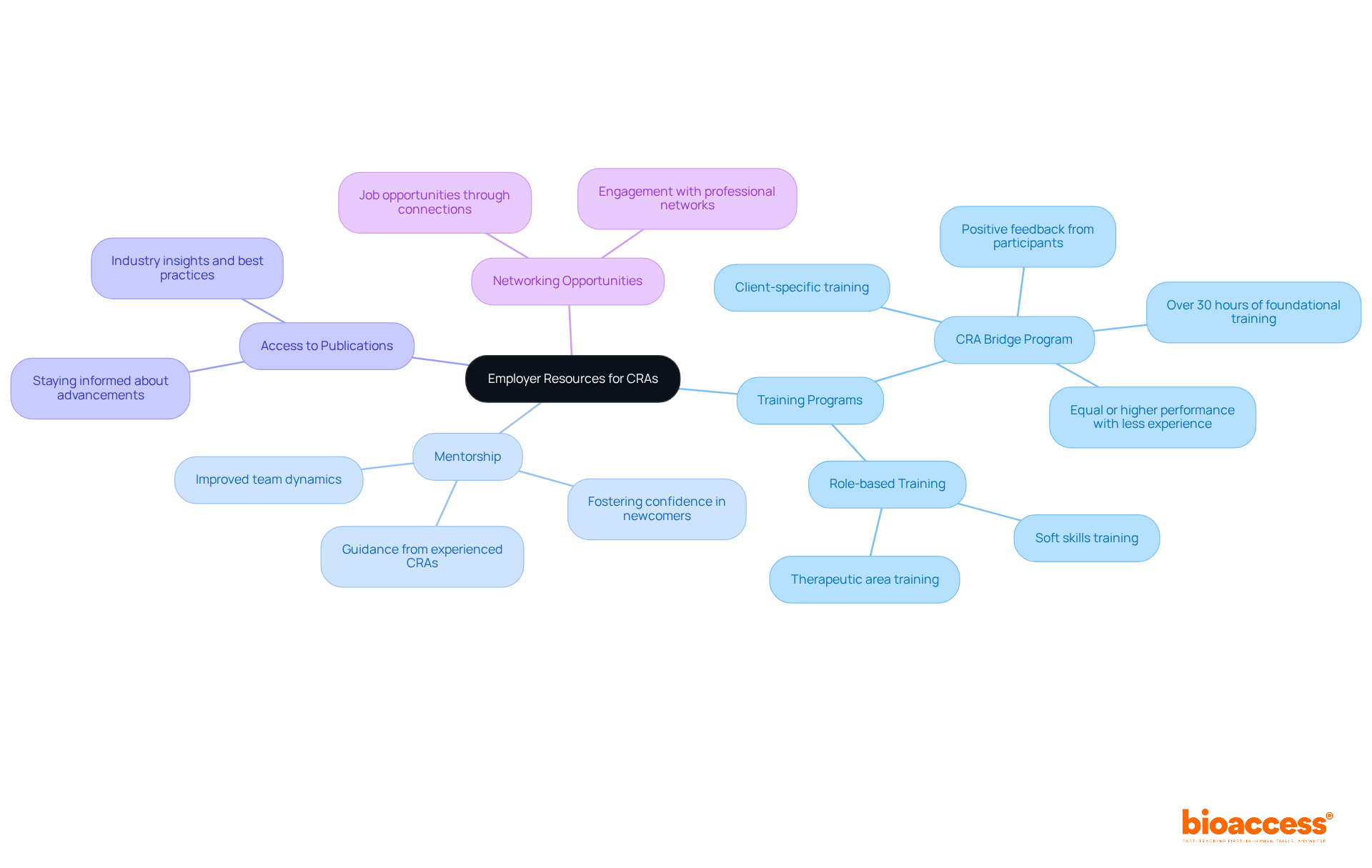
Employers play a pivotal role in equipping Clinical Research Associates for senior CRA jobs with essential resources, significantly enhancing their professional capabilities. Comprehensive training programs are vital for senior CRA jobs; they not only keep clinical research associates informed about regulatory changes and best practices but also promote a culture of ongoing enhancement. For instance, structured training initiatives, such as the CRA Bridge Program, have demonstrated that participants often achieve equal or higher performance levels compared to their more experienced counterparts, even with less than two years of experience. Since 2013, over 3,000 participants have successfully completed the CRA Bridge Program, underscoring its success and credibility.
In addition to formal training, mentorship programs are invaluable for facilitating knowledge sharing and professional growth. Experienced research professionals can assist newcomers, helping them navigate the intricacies of trials and fostering confidence in their positions. This mentorship not only enhances the effectiveness of clinical research associates but also contributes to a positive work environment, as evidenced by case studies highlighting improved team dynamics and reduced turnover rates.
Furthermore, access to industry publications and networking opportunities enables CRAs to remain informed about the latest advancements in research. Engaging with professional networks can lead to senior CRA jobs and provide support from experienced professionals in the field. By investing in these resources, employers can cultivate a highly skilled and engaged workforce, ultimately leading to more successful project outcomes and a stronger organizational culture.
Job advertisements for senior CRA jobs typically outline prerequisites such as:
Employers actively seek candidates with:
Given Bioaccess's extensive clinical trial management services—which encompass:
Candidates should emphasize their experience in these critical areas. Recognizing these elements enables candidates to effectively tailor their resumes and cover letters to meet employer expectations, particularly within organizations dedicated to enhancing global health through international collaboration and innovation in medtech.
Senior CRA jobs offer numerous advantages, including competitive salaries typically ranging from $60,000 to $80,000 for entry-level positions. These roles provide significant opportunities for professional development, with many organizations granting access to certifications such as the Certified Clinical Research Professional (CCRP) and the Certified Clinical Research Associate (CCRA). Such credentials not only enhance career prospects but also equip professionals with essential skills in a rapidly evolving field.
Flexibility stands out as another key advantage; many clinical research associates value remote work options that significantly improve work-life balance. This flexibility correlates positively with job satisfaction, as studies reveal that employees who can manage their schedules often report higher levels of fulfillment in their roles.
Furthermore, clinical research associates play a crucial role in advancing medical knowledge, engaging in groundbreaking research that directly impacts patient care. This sense of purpose resonates with numerous experts in the field, who derive satisfaction from their contributions to research studies and the development of new treatments. As the medical research landscape evolves, senior CRA jobs become increasingly vital, offering not merely a job but a meaningful career path.
High-paying senior CRA jobs predominantly exist within specialized therapeutic areas such as oncology, cardiology, and rare diseases. These roles often necessitate advanced degrees and substantial experience in medical research, with salaries that reflect the specialized knowledge required. For example, the median annual salary for senior clinical research associates is reported at $126,837, with top-paying states like California offering even higher compensation.
Candidates can significantly enhance their earning potential by pursuing relevant certifications and acquiring experience in high-demand therapeutic areas. The career trajectory for senior CRA jobs is promising, with a projected growth rate of 6% over the next decade, especially in oncology, where the demand for skilled professionals is increasing.
Networking within the industry is essential for uncovering lucrative job opportunities. Industry leaders emphasize the increasing demand for senior CRA jobs in oncology and rare diseases, underscoring the rewarding nature of these careers. As the pharmaceutical landscape evolves, CRAs who specialize in these areas will find themselves at the forefront of clinical research, contributing to groundbreaking advancements in treatment and patient care.
The landscape of senior clinical research associate (CRA) jobs is evolving rapidly, presenting numerous opportunities for career advancement in 2025. This article highlights various positions across specializations such as oncology, diabetes, and ophthalmology, underscoring the critical roles CRAs play in ensuring compliance, patient safety, and effective study management. The demand for skilled CRAs is on the rise, driven by the increasing complexity of clinical trials and the need for rigorous oversight in research practices.
Key insights from the discussion include:
Additionally, the transition from clinical research coordinators to home-based CRAs is a viable pathway for career growth, emphasizing the need for networking and mentorship within the field. As the job market continues to grow, understanding these dynamics will empower professionals to navigate their career paths effectively.
In conclusion, pursuing a career as a senior CRA offers not only competitive salaries and flexible work arrangements but also the opportunity to contribute meaningfully to advancements in healthcare. As the industry adapts to new challenges and opportunities, aspiring CRAs should actively seek out resources, training, and networking opportunities to position themselves for success. Embracing these strategies will enhance personal career trajectories and play a pivotal role in shaping the future of clinical research.
What is the role of a Senior Clinical Research Associate (CRA) at bioaccess® in oncology?
The Senior CRA in oncology at bioaccess® is responsible for managing oncology investigations, ensuring compliance with regulatory standards, and monitoring patient safety and data integrity.
What are the main responsibilities of a Senior CRA in oncology?
Key responsibilities include supervising research implementation, conducting feasibility assessments, site selection, compliance reviews, project management, and reporting on research status and adverse events.
Why is compliance important in oncology clinical research?
Compliance is crucial in oncology research due to the high occurrence of serious adverse events (SAEs) and the need for rigorous oversight to maintain data integrity and participant safety.
What trends are emerging in oncology clinical research?
There is a growing emphasis on compliance and proactive management strategies, which have been shown to improve patient recruitment and retention rates, enhancing overall study outcomes.
What is the focus of Senior CRA jobs for diabetes on the West Coast?
These remote positions focus on overseeing studies aimed at enhancing diabetes management and treatment, while ensuring compliance with Good Clinical Practice (GCP) guidelines and addressing challenges in patient recruitment and retention.
What unique challenges do Senior CRAs face in CNS and ophthalmology research?
CRAs in CNS and ophthalmology must navigate significant hurdles related to patient recruitment and retention, including stringent eligibility criteria and misconceptions about research risks.
How does bioaccess® address recruitment challenges in CNS and ophthalmology studies?
Bioaccess® employs accelerated patient recruitment and site activation services, utilizing pre-qualified networks and compliant datasets to streamline the recruitment process.
What partnerships have demonstrated success in improving recruitment and retention in clinical trials?
Partnerships like that of GlobalCare Clinical Trials with bioaccess® have shown the potential to reduce recruitment time by over 50% and achieve retention rates exceeding 95%.