


Class 1 medical devices form the backbone of healthcare technology, encompassing a wide range of everyday items that are crucial for patient care. Remarkably, about 35% of all regulated medical devices fall into this category, making it essential for healthcare professionals and manufacturers to grasp their definitions, regulatory context, and impact. As the demand for these devices continues to rise, the challenges surrounding compliance and safety standards also intensify.
How can the industry ensure that these vital tools remain accessible while adhering to the highest safety protocols?
Category 1 healthcare products represent the lowest risk classification within the healthcare product classification system. These instruments are typically non-invasive and designed without the intent to support or sustain life. The FDA categorizes around 35% of all regulated medical devices as class 1 devices, underscoring their prevalence in healthcare. General controls govern these instruments, which include essential requirements such as registration, labeling, and adherence to Good Manufacturing Practices (GMP). Notably, approximately 93% of Category 1 products are exempt from premarket notification obligations, providing a more straightforward path to market entry compared to higher-risk categories.
Examples of class 1 devices include everyday items like:
While these devices are vital for patient care and safety, they often encounter compliance challenges. Alarmingly, about 76% of healthcare devices lack crucial labeling information, highlighting the urgent need for manufacturers to prioritize compliance. As industry experts assert, "The complexity of medical equipment regulation necessitates a proactive compliance strategy, ensuring that unsafe or ineffective products do not reach the market."
Recent updates from the FDA indicate a continued focus on enhancing regulatory frameworks for class 1 devices. For instance, by May 26, 2025, all Category 1 product labels and outer packaging must feature a Unique Product Identifier (UDI) in both barcode and human-readable formats. This requirement aims to bolster traceability and safety, emphasizing the critical importance of compliance in the healthcare equipment landscape.
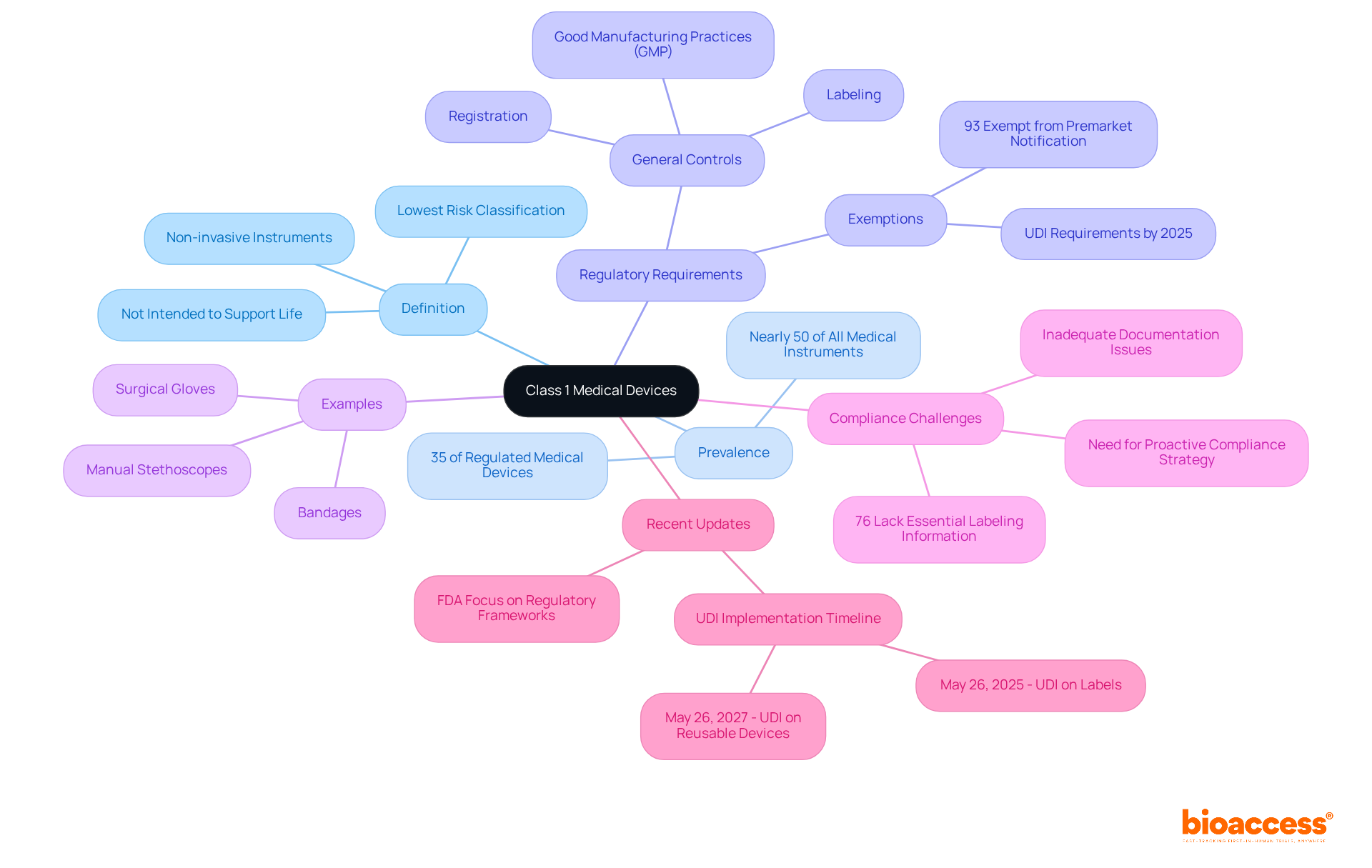
Category 1 medical products are regulated under the Federal Food, Drug, and Cosmetic Act in the United States, where the FDA categorizes medical products into three groups based on risk: Category 1, Category 2, and Category 3. As the lowest risk category, Group 1 products face the least stringent regulatory controls. Notably, most Category 1 products are exempt from the premarket notification procedure (510(k)), which is mandatory for Category 2 and Category 3 items. This exemption significantly accelerates market entry, enabling manufacturers to swiftly meet pressing healthcare needs. For instance, the FDA has recognized numerous Category 1 products, including latex gloves and surgical masks, that do not require 510(k) submissions, facilitating their rapid availability in the market.
In the European Union, Category 1 products are similarly classified under the Medical Product Regulation (MPR), reinforcing their low-risk status and the general controls governing their marketing and usage. According to FDA representatives, these general controls are vital for ensuring safety and effectiveness while allowing a streamlined pathway for class 1 devices to reach consumers. In Colombia, the oversight framework is managed by INVIMA, the National Food and Drug Surveillance Institute, which plays a crucial role in examining and monitoring health products, including healthcare instruments. With specialists like Ana Criado, who possesses extensive experience in compliance matters and biomedical engineering, and Katherine Ruiz, who focuses on compliance for health-related tools and in vitro diagnostics, the Colombian market is well-equipped to adhere to international standards. This governing structure not only fosters innovation but also enhances patient access to essential healthcare products.
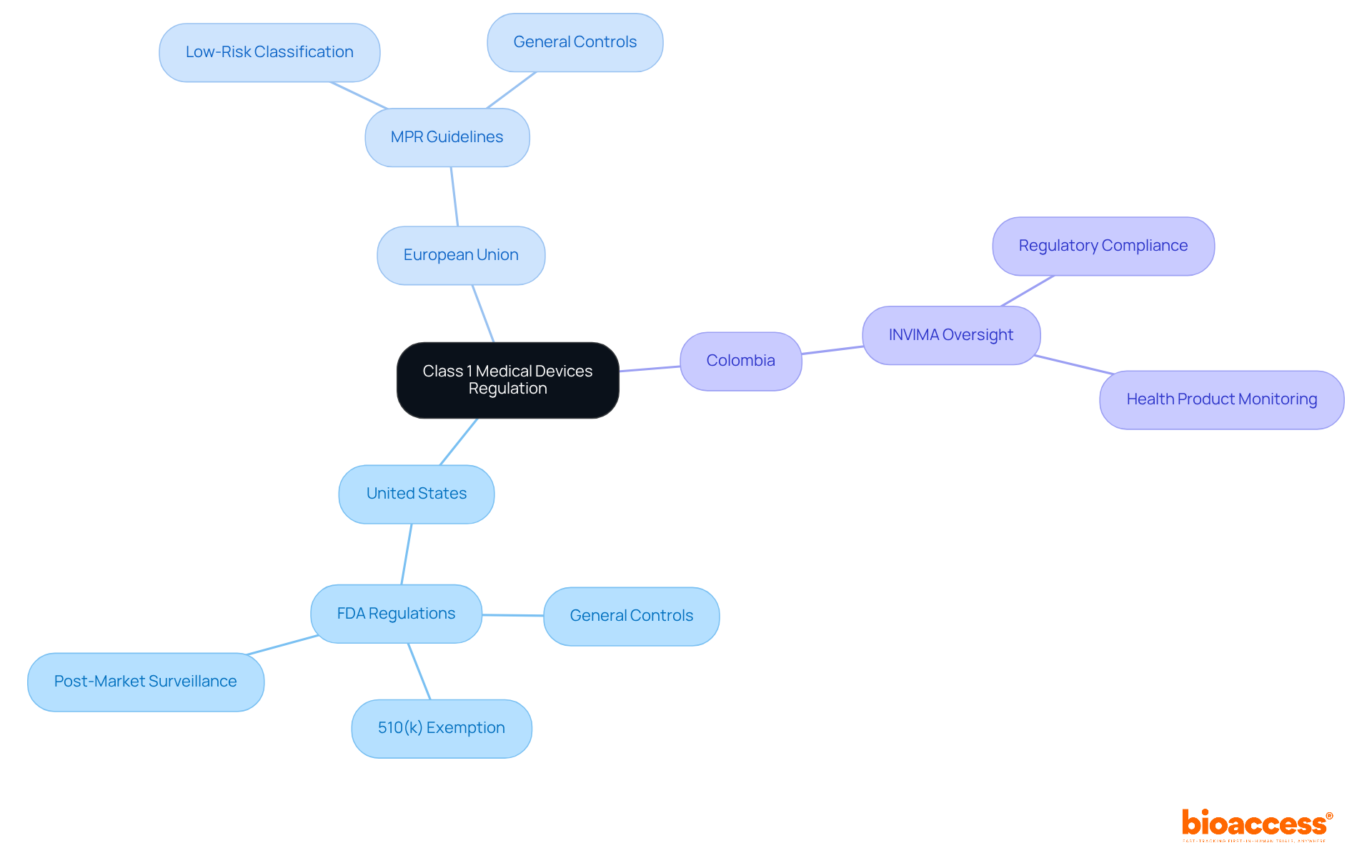
Class 1 devices play a crucial role in daily healthcare, characterized by their low risk and minimal patient interaction. These vital tools not only facilitate patient care but also demand less regulatory scrutiny, allowing for quicker market entry. Common examples include:
The market for Category 1 products is substantial, representing a significant portion of the overall healthcare equipment market. As healthcare continues to evolve, the demand for class 1 devices remains robust, underscoring their importance in delivering safe and effective patient care. Healthcare experts emphasize that the reliability and availability of class 1 devices are essential for facilitating daily healthcare practices, ultimately enhancing patient outcomes. Furthermore, the regulatory framework surrounding Category 1 instruments supports an efficient approval process, crucial for meeting the urgent needs of healthcare providers and patients alike. Industry reports indicate that Category 1 instruments account for approximately 40% of the overall healthcare equipment market, highlighting their pivotal role in healthcare innovation.
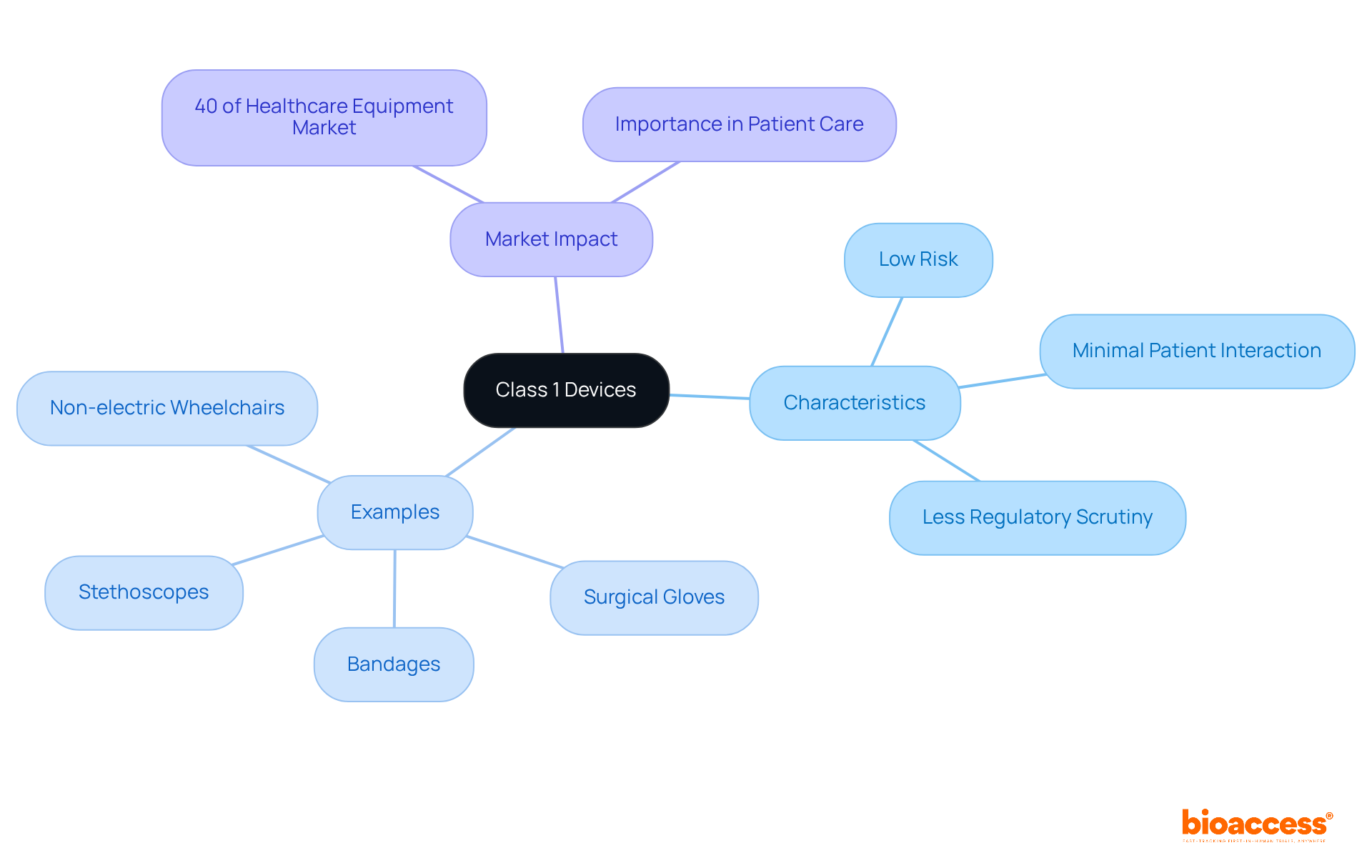
Category 1 medical instruments play a crucial role in medical research, especially in early-phase clinical trials. Their classification as low-risk allows researchers to pursue innovative solutions without the heavy regulatory hurdles faced by higher-risk products. For example, the swift endorsement and implementation of Category 1 instruments can greatly enhance the efficiency of clinical trials, leading to quicker data collection and analysis. In fact, statistics reveal that these tools are vital to about 70% of early-phase studies, highlighting their importance in accelerating research timelines.
Moreover, the accessibility of Category 1 tools fosters collaboration among researchers and manufacturers, creating a dynamic environment for innovation. bioaccess™ has been instrumental in supporting Avantec Vascular by assisting in the selection of a principal investigator and the submission of regulatory dossiers for their first-in-human clinical study of a groundbreaking vascular instrument in Latin America. Clinical researchers have noted that the use of class 1 devices enhances immediate patient care and aligns with the broader objectives of healthcare research by facilitating the development of new technologies and treatment methodologies.
This synergy ultimately speeds up the transition from concept to clinical application, benefiting both the medical community and patients alike. As we look ahead, the importance of collaboration in this field cannot be overstated, and the next steps involve leveraging these tools to further advance medical research.
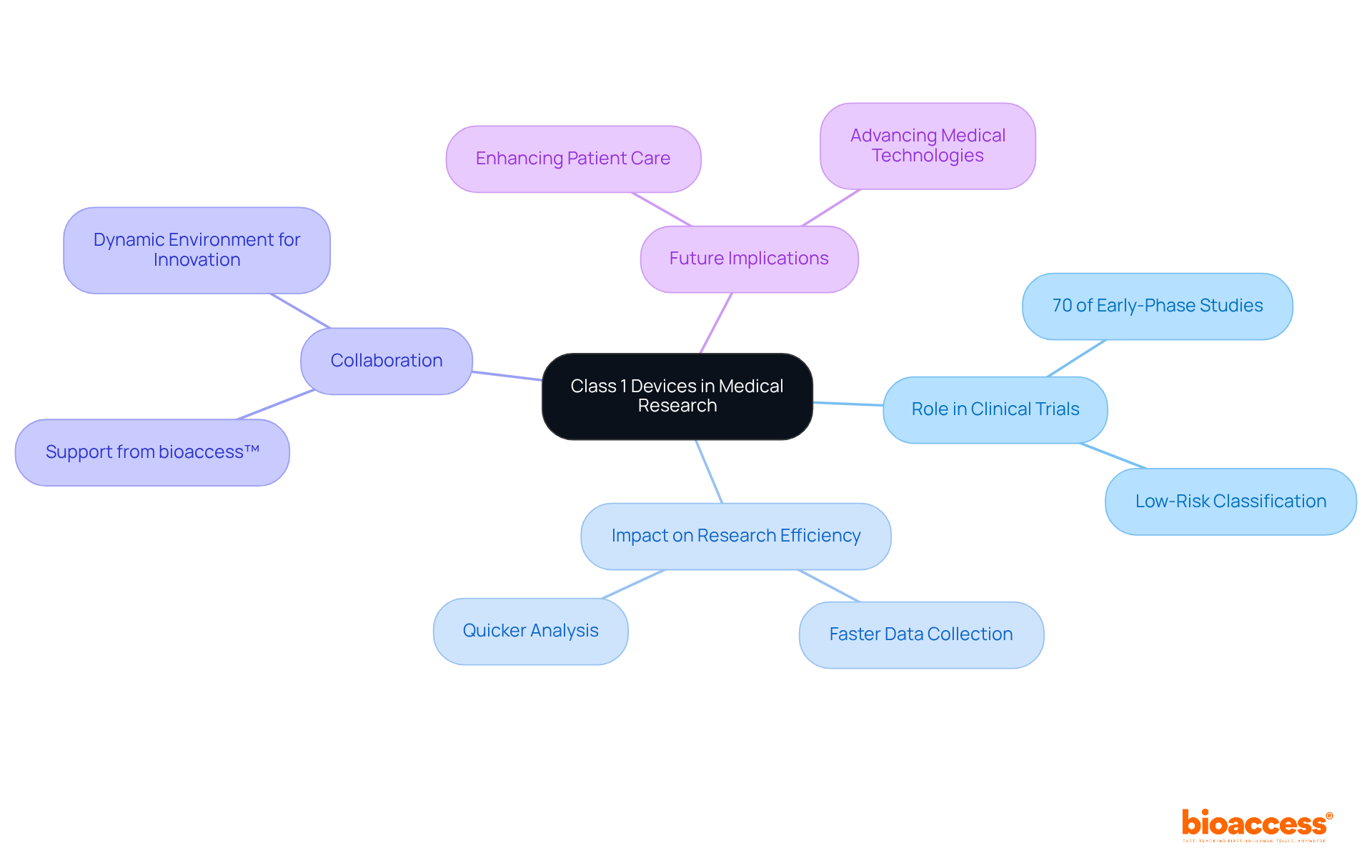
Class 1 medical devices serve as foundational elements in the healthcare landscape, categorized as low-risk products that are vital for patient care and safety. Their non-invasive nature and streamlined regulatory pathway facilitate quicker access to essential healthcare tools, crucial for both everyday medical practices and innovative research initiatives.
The significance of Class 1 devices is underscored by their prevalence in the medical market, with examples ranging from bandages to surgical gloves. Regulatory frameworks governing these devices are critical, emphasizing the importance of compliance and the challenges faced in ensuring proper labeling and safety standards. Notably, Class 1 devices play a pivotal role in facilitating medical research and clinical trials, allowing for rapid innovation and improved patient outcomes.
In summary, the impact of Class 1 medical devices extends beyond their immediate utility; they are integral to advancing healthcare technology and research. As the regulatory landscape evolves, prioritizing compliance and innovation is essential for harnessing the full potential of these devices. Stakeholders in the healthcare sector must remain vigilant in their efforts to enhance product safety and efficacy, ultimately improving patient care and supporting ongoing medical advancements.
What are Class 1 medical devices?
Class 1 medical devices are healthcare products that represent the lowest risk classification. They are typically non-invasive and not intended to support or sustain life.
What percentage of regulated medical devices are classified as Class 1?
Approximately 35% of all regulated medical devices are categorized as Class 1 devices.
What types of controls govern Class 1 medical devices?
Class 1 medical devices are governed by general controls, which include requirements such as registration, labeling, and adherence to Good Manufacturing Practices (GMP).
Are Class 1 medical devices usually exempt from premarket notification?
Yes, about 93% of Class 1 products are exempt from premarket notification obligations, making their path to market entry simpler compared to higher-risk categories.
Can you provide examples of Class 1 medical devices?
Examples of Class 1 medical devices include bandages, surgical gloves, and manual stethoscopes.
What compliance challenges do Class 1 medical devices face?
Many Class 1 medical devices encounter compliance challenges, with about 76% lacking crucial labeling information.
What recent updates have been made regarding Class 1 medical device labeling?
By May 26, 2025, all Class 1 product labels and outer packaging must include a Unique Product Identifier (UDI) in both barcode and human-readable formats to enhance traceability and safety.
Why is compliance important for Class 1 medical devices?
Compliance is critical to ensure that unsafe or ineffective products do not reach the market, highlighting the need for a proactive compliance strategy in the healthcare equipment landscape.