


Understanding the landscape of clinical trial registries is crucial for advancing medical research, particularly in regions like Montenegro. These registries enhance transparency and accountability, playing a pivotal role in fostering public trust and ethical practices in clinical studies. However, navigating the complex requirements set forth by regulatory bodies can pose significant challenges for researchers. This raises important questions about the balance between compliance and innovation.
What does it truly take to successfully register a clinical trial in Montenegro? How do these requirements shape the future of medical research in the region? As we delve deeper into this topic, we will explore the Medtech landscape and the role of bioaccess in addressing key challenges faced by researchers.
In summary, collaboration among stakeholders is essential for overcoming these hurdles and ensuring the integrity of clinical research in Montenegro.
Medical study registries serve as essential public databases that catalog ongoing and completed studies, significantly enhancing transparency in medical research. By meticulously documenting study objectives, methodologies, and outcomes, these registries effectively combat publication bias and promote accountability among researchers. They ensure that participants are thoroughly informed about the trials they may consider joining, thereby upholding ethical standards in research.
In Montenegro, the clinical trial registry requirements are significant as they extend beyond mere compliance and are pivotal in building public trust in medical research activities. Successful examples from various jurisdictions demonstrate how robust registry practices can lead to improved patient outcomes and notable advancements in medical science. Furthermore, the transparency afforded by these registries facilitates the exchange of knowledge and results, ultimately contributing to a more ethical and accountable research environment.
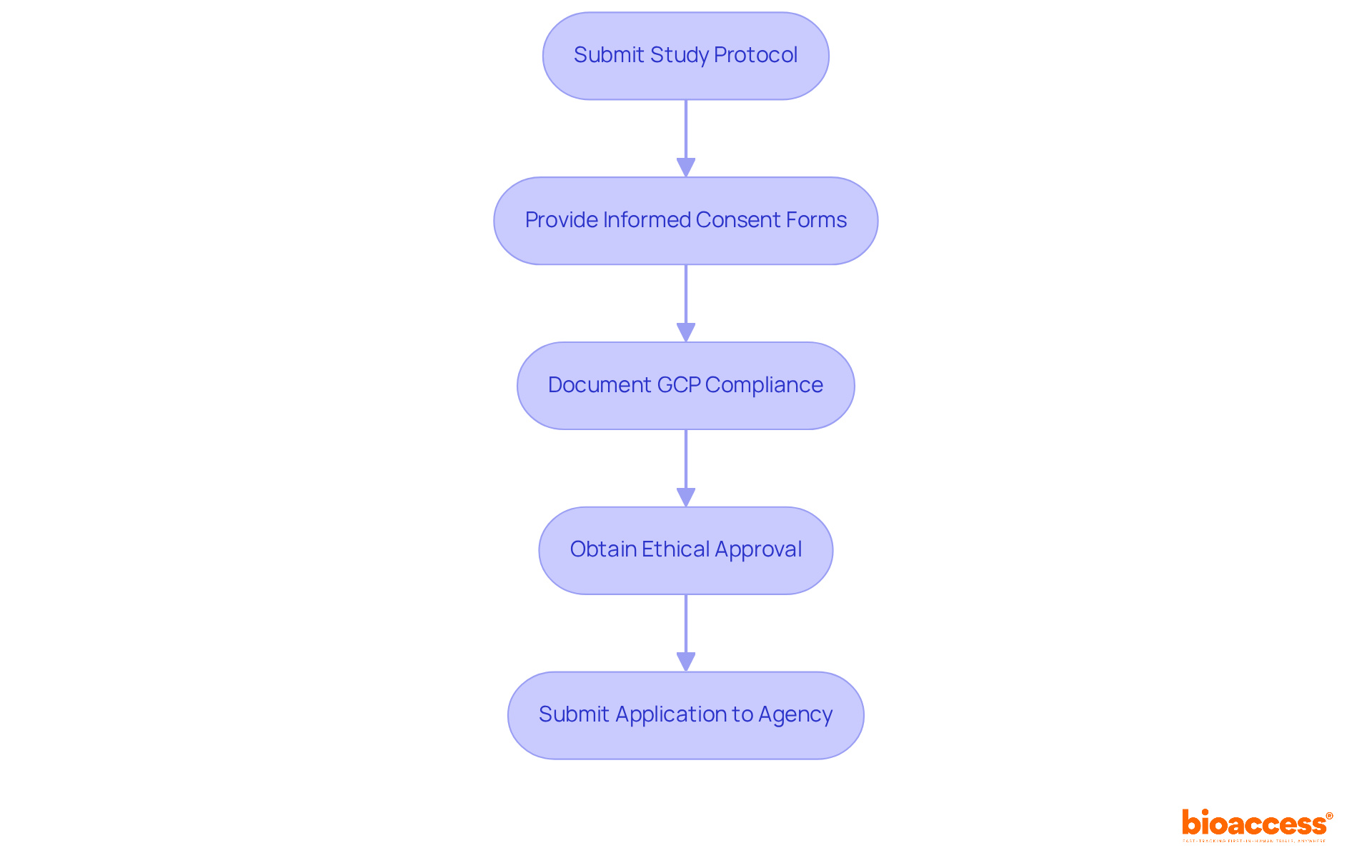
In Montenegro, the clinical trial registry requirements in Montenegro are dictated by the Law on Medicines and the Law on Clinical Research. Essential requirements, including compliance with clinical trial registry requirements in Montenegro, involve submitting a detailed study protocol, informed consent forms, and documentation demonstrating adherence to Good Clinical Practice (GCP) guidelines. To comply with the clinical trial registry requirements in Montenegro, sponsors must also provide proof of ethical approval from a recognized ethics committee before submitting their application to the Agency for Medicines and Medical Devices. This registration process involves a thorough examination by governing bodies to ensure compliance with the clinical trial registry requirements in Montenegro, protecting participants' rights and safety while promoting high-quality study outcomes.
Recent updates to the Clinical Trials Rulebook have refined the criteria for conducting Phase I studies, enhancing the oversight framework. Adherence to GCP is not merely a legal requirement; it is crucial for preserving public confidence in medical studies. Trials that follow these guidelines are significantly more likely to have their data accepted by oversight bodies, facilitating the approval process for new therapies. However, startups often encounter challenges such as protocol adherence and documentation issues, highlighting the necessity for thorough preparation.
bioaccess® offers accelerated site activation in under 8 weeks, providing FDA/EMA/MDR-ready datasets with centralized monitoring. This capability is particularly advantageous for startups navigating the intricate regulatory demands of early-stage studies. bioaccess®'s expertise in optimizing procedures helps overcome the obstacles associated with prolonged approval timelines, ensuring a smoother path to success.
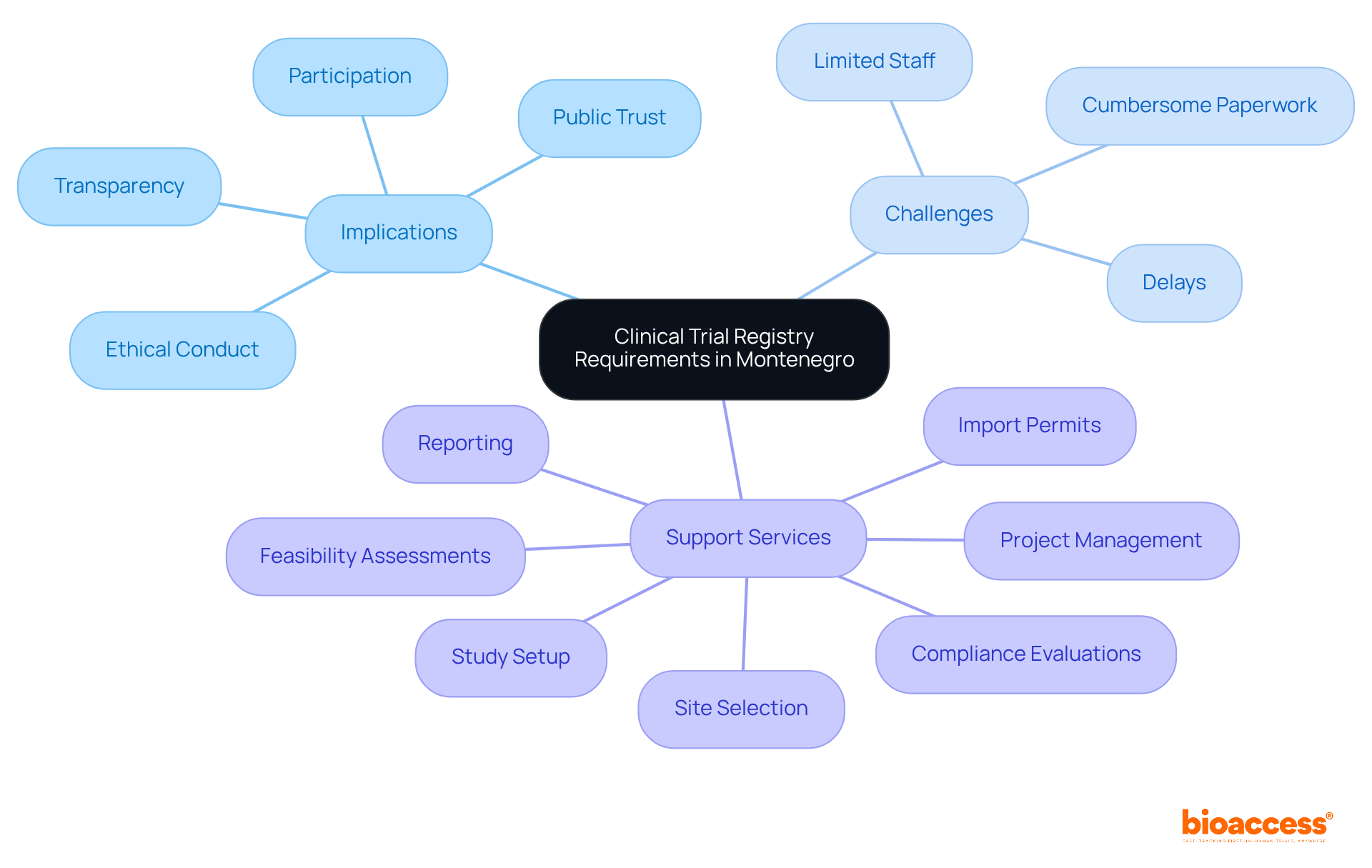
The clinical trial registry requirements in Montenegro are crucial in shaping the landscape of medical studies. By mandating transparency and ethical conduct, these regulations not only enhance the integrity of research but also foster public trust and encourage participation in clinical studies. However, the stringent nature of the clinical trial registry requirements in Montenegro presents significant challenges for researchers. The time and resources needed to manage compliance demands often lead to heightened administrative burdens, with 74% of investigators citing limited staff as a major barrier and 71% noting cumbersome paperwork as a significant concern. Such challenges can result in delays, as compliance with complex protocols may extend timelines and complicate study designs.
Moreover, the focus on meeting compliance standards can influence the types of studies undertaken. Researchers may find themselves prioritizing trials that closely align with compliance expectations, which could inadvertently restrict the exploration of innovative approaches that might benefit patient populations. While the registration procedure is essential for safeguarding participants and ensuring high-quality clinical investigations, it also introduces complexities that researchers must adeptly navigate to advance their studies efficiently.
In Montenegro, the challenges of adhering to the clinical trial registry requirements are further complicated by the necessity for robust ethical oversight, often leading to protocol amendments and additional studies. The interplay between regulatory demands and the practicalities of conducting studies underscores the importance of efficient procedures and support mechanisms. Bioaccess offers extensive research study management services, including:
These services can significantly alleviate the burden on researchers. With overall success rates for drug development estimated to be under 12-15% and median Phase III enrollment durations increasing from approximately 13 to 18 months, the research environment remains fraught with challenges that require careful navigation.
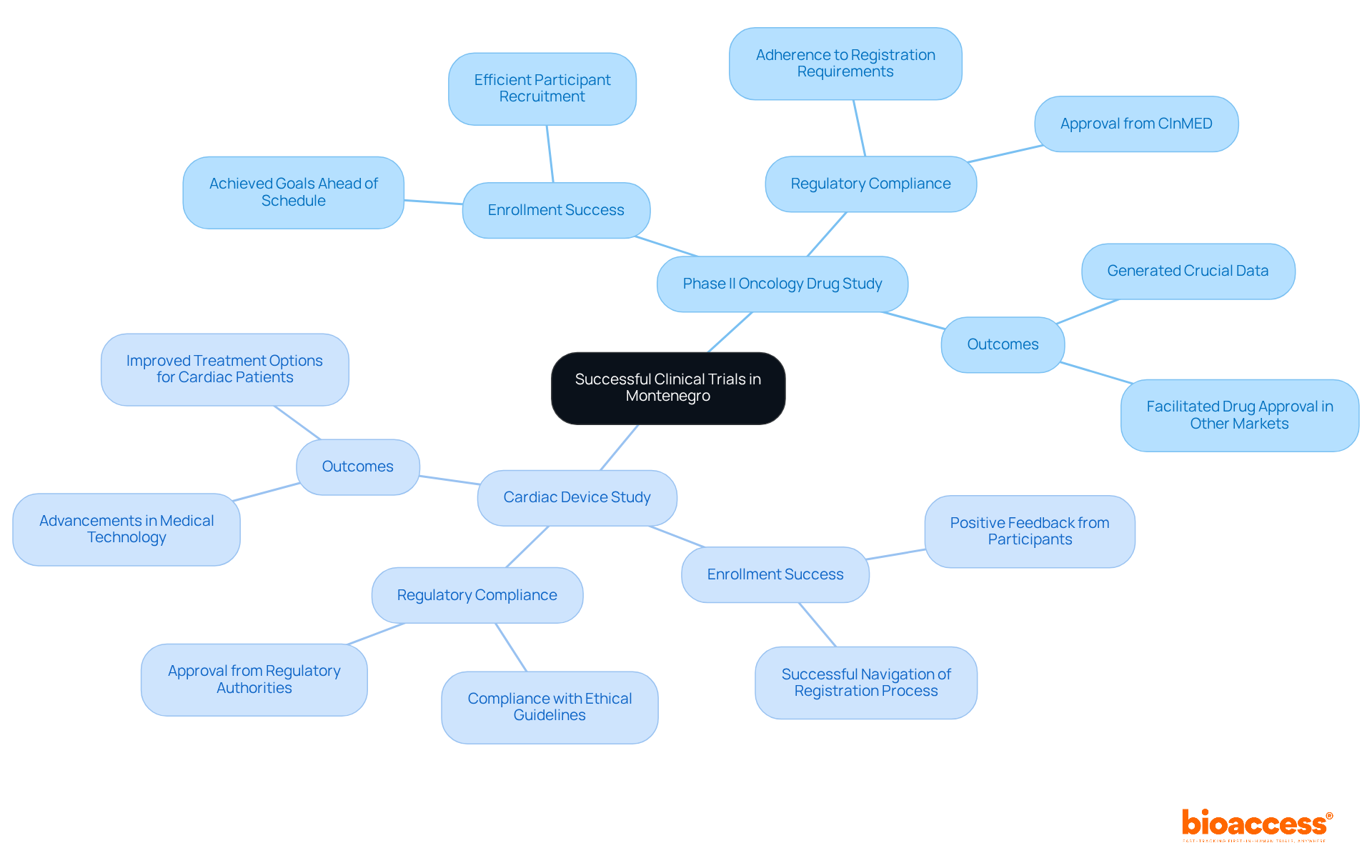
Montenegro has witnessed remarkable success in clinical studies, showcasing the strength of its regulatory framework. A notable recent phase II study for a novel oncology drug exemplifies local researchers' ability to efficiently recruit participants while strictly adhering to registration requirements. This study not only achieved its enrollment goals ahead of schedule but also generated crucial data that facilitated the drug's approval in other markets.
Additionally, a research study focused on a new medical device for cardiac patients successfully navigated the registration process, garnering positive feedback from both participants and regulatory authorities. These examples illustrate how compliance with registration requirements can lead to successful outcomes, driving advancements in medical technology and treatment options within Montenegro.
Organizations like bioaccess® play a pivotal role in these early-phase oncology studies, ensuring that innovative treatments reach patients swiftly. Their comprehensive clinical trial management services - including feasibility studies, site selection, compliance reviews, trial setup, import permits, project management, and reporting - position bioaccess® as a leader in medtech clinical research in Latin America. This leadership is further exemplified by their collaboration with Avantec Vascular for a first-in-human study.
The significance of clinical trial registries in Montenegro is paramount, as they enhance transparency and accountability within medical research. By adhering to established registry requirements, researchers uphold ethical standards and foster public trust - an essential element for successful participant recruitment and the advancement of medical science.
Key elements such as the legal framework governing clinical trials, the challenges researchers face in meeting compliance standards, and the successful outcomes of various studies illustrate the complexity and necessity of these registries. Regulatory requirements ensure that trials are conducted ethically and transparently, while also emphasizing the importance of support services that alleviate administrative burdens on researchers.
Looking at the broader implications, it’s evident that the landscape of clinical research in Montenegro is evolving. As the regulatory framework develops, stakeholders must advocate for streamlined processes that balance compliance with innovation. This approach can unlock the potential for groundbreaking medical advancements, ultimately benefiting public health and enhancing the integrity of clinical research in the region.
What are clinical trial registries?
Clinical trial registries are public databases that catalog ongoing and completed medical studies, documenting their objectives, methodologies, and outcomes.
Why are clinical trial registries important?
They enhance transparency in medical research, combat publication bias, promote accountability among researchers, and ensure that participants are informed about the trials they may consider joining.
How do clinical trial registries uphold ethical standards in research?
By providing comprehensive information about trials, registries ensure that potential participants are well-informed, which helps maintain ethical standards in research.
What is the significance of clinical trial registry requirements in Montenegro?
In Montenegro, these requirements are crucial for building public trust in medical research activities and go beyond mere compliance.
How can robust registry practices impact patient outcomes?
Successful examples from various jurisdictions show that strong registry practices can lead to improved patient outcomes and advancements in medical science.
What role do clinical trial registries play in knowledge exchange?
The transparency provided by registries facilitates the exchange of knowledge and results, contributing to a more ethical and accountable research environment.