


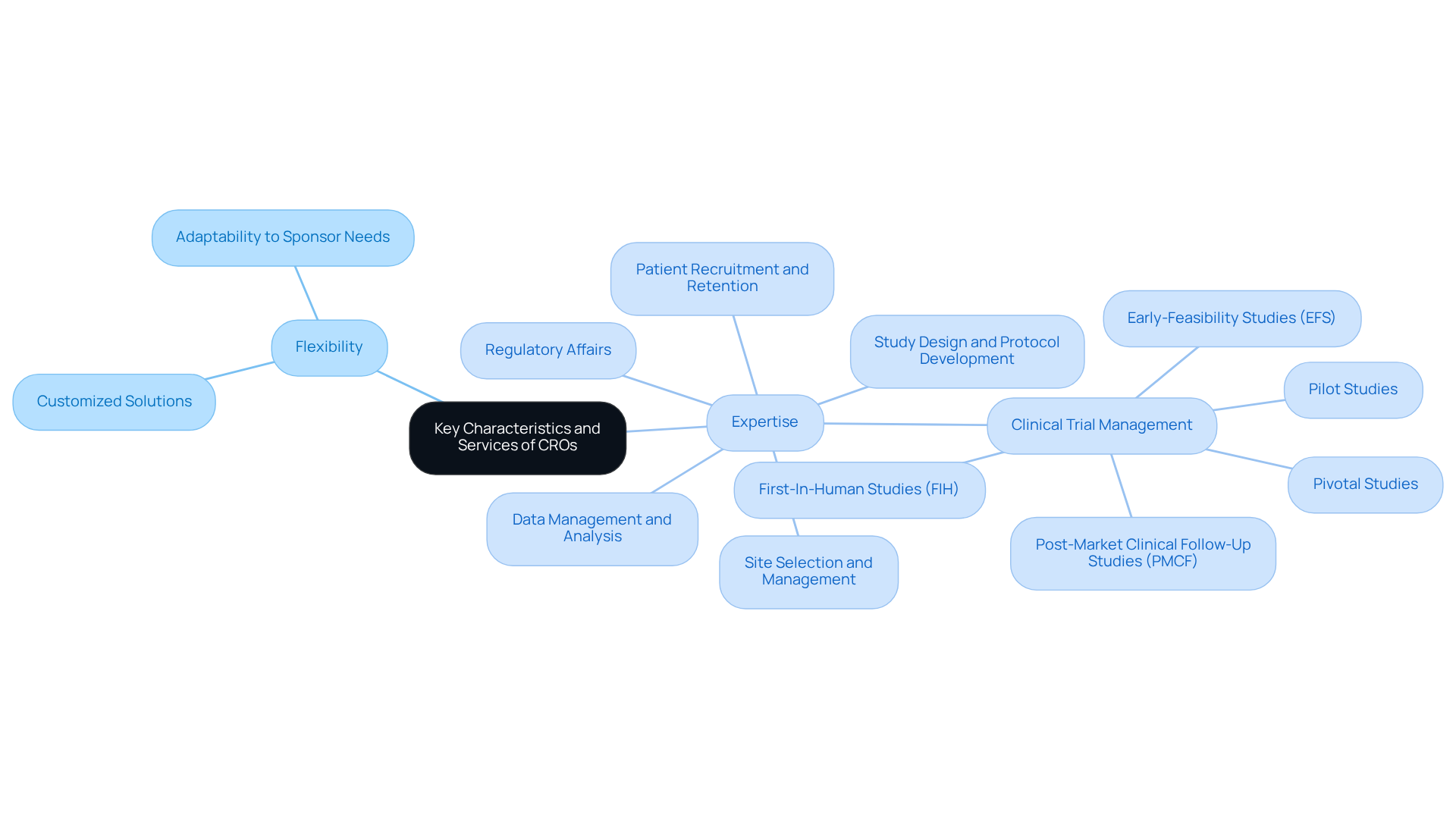
Contract Research Organizations (CROs) serve as specialized entities that deliver essential outsourced services to the pharmaceutical, biotechnology, and medical device sectors. They manage critical aspects of clinical trials, including:
This article underscores their growing significance in the research landscape, revealing that CROs conduct nearly 75% of clinical studies worldwide. By leveraging advanced technologies and strategic partnerships, CROs facilitate faster and more efficient drug development processes, addressing the challenges faced by the industry.
Contract Research Organizations (CROs) play a pivotal role in the intricate landscape of clinical trials, acting as the essential link between innovative drug development and regulatory compliance. By offering a comprehensive suite of specialized services—from study design to patient recruitment—CROs empower pharmaceutical and biotechnology companies to streamline their research processes, allowing them to concentrate on their core competencies.
As the industry evolves and the demand for rapid, efficient clinical trials intensifies, it raises a critical question: how can CROs adapt to meet the challenges of an ever-accelerating market?
This article explores the significant role, historical evolution, and future trends of CROs, revealing the profound impact they have on the success of clinical research.
Contract research organizations (CROs) serve as specialized entities that provide outsourced services to the pharmaceutical, biotechnology, and medical device sectors. Anticipated to oversee a substantial part of research studies in 2025, contract research organizations (CROs) emphasize their essential role within the sector. These organizations manage various elements of clinical studies, including:
By offering these vital services, contract research organizations allow sponsors to focus on their primary strengths while making sure that trials are conducted effectively and comply with legal standards.
Contract research organizations (CROs) play a pivotal role in the drug development process, facilitating the transition from research to market by delivering the necessary expertise and resources. For instance, bioaccess® exemplifies a CRO that accelerates research by leveraging regulatory speed in Latin America and diverse patient pools. This enables ethical approvals in just 4-6 weeks and achieves enrollment rates that are 50% faster than traditional markets.
Recent advancements in CRO services have involved the implementation of digital data collection technologies and enhanced patient engagement strategies, including the utilization of wearables in studies. These innovations not only enhance data precision but also simplify procedures. As the contract research organizations market continues to evolve, the significance of these organizations in research studies remains crucial, fostering innovation and efficiency within the pharmaceutical and biotechnology sectors.
Contract Research Entities (CROs) play a vital role in the research landscape, serving as key collaborators that connect sponsors with the intricate network of compliance requirements. They offer a comprehensive range of services, including:
This streamlines trial operations from initial planning to final reporting. By leveraging their specialized knowledge, contract research organizations help sponsors navigate the complexities of medical research, ensuring adherence to ethical standards and regulatory guidelines. This partnership not only accelerates the development of innovative treatments but also enhances the quality and reliability of research data.
For instance, a European biotech firm successfully reduced its time-to-offer from over 50 days to just 36 by collaborating with a CRO, showcasing the efficiency gains that such partnerships can achieve. Notably, contract research organizations conduct nearly 75% of clinical studies worldwide, underscoring their essential role in advancing medical knowledge and improving patient outcomes. As the industry evolves, there is a growing reliance on contract research organizations collaborations, especially in managing the compliance landscape and enhancing study effectiveness—both critical for the successful marketing of new treatments.
bioaccess® distinguishes itself by delivering ethical approvals in just 4-6 weeks and achieving enrollment 50% faster than traditional markets, presenting a unique value proposition for Medtech and Biopharma innovators. This capability is crucial as drug developers encounter challenges such as patient recruitment and regulatory hurdles, making the expertise of contract research organizations like bioaccess® invaluable.
Furthermore, bioaccess® has partnered with the Caribbean Health Group to position Barranquilla as a leading hub for medical studies in Latin America, supported by Colombia's Minister of Health. The recent first-in-human study of Flow-FX's innovative Flow-Screw device for intraosseous antibiotic delivery in Colombia exemplifies this fruitful collaboration. Additionally, GlobalCare Clinical Trials has joined forces with bioaccess® to enhance ambulatory services for studies in Colombia, achieving over a 50% reduction in recruitment duration and impressive 95% retention rates. Dushyanth Surakanti, Founder & CEO of Sparta Biomedical, has shared his positive experiences with bioaccess® during its initial human study in Colombia, further emphasizing the effectiveness of these collaborations.

The origins of Contract Research Entities can be traced back to the mid-20th century, a pivotal time when pharmaceutical firms began outsourcing specific aspects of research studies to enhance cost control and operational effectiveness. Initially, contract research organizations focused on data management and monitoring. However, as the industry evolved, their service offerings expanded significantly. Today, contract research organizations offer a comprehensive array of solutions, including compliance matters, site administration, and patient recruitment, demonstrating a holistic approach to research.
The transformation of CRO services has been propelled by globalization and rapid technological advancements. These factors have enabled clinical research organizations to operate across diverse regions, such as Latin America, the Balkans, and Australia, leveraging local regulatory efficiencies and patient diversity to optimize study processes. For example, bioaccess® exemplifies this evolution by securing ethical approvals in a mere 4-6 weeks and facilitating patient enrollment that is 50% faster than traditional markets. This strategic flexibility not only accelerates the clinical study process but also enhances the overall efficiency of experiments.
Furthermore, the integration of advanced technologies, such as electronic data capture and telehealth solutions, has revolutionized how contract research organizations conduct trials. These innovations significantly improve patient engagement and streamline data collection, effectively addressing the challenges posed by regulatory delays and recruitment hurdles. As the landscape of contract research organizations evolves, the emphasis on providing comprehensive, technology-driven solutions remains paramount, ensuring that these organizations can meet the growing demands of the pharmaceutical and biopharmaceutical sectors.

Recognized for their flexibility, expertise, and ability to provide customized solutions, contract research organizations meet the distinct needs of sponsors. Among the essential services offered by CROs are:
These comprehensive services empower contract research organizations, such as bioaccess®, to assist sponsors in accelerating the market introduction of new therapies, particularly within the multi-billion dollar healthcare market of Latin America. With over 20 years of experience in Medtech, bioaccess® exemplifies this commitment by delivering ethical approvals in just 4-6 weeks and achieving participant enrollment 50% faster than traditional markets.
Contract research organizations (CROs) play a pivotal role in the clinical trial landscape, delivering essential services that significantly enhance the efficiency and effectiveness of drug development processes. By outsourcing critical functions such as study design, patient recruitment, and regulatory compliance to CROs, pharmaceutical and biotechnology companies can concentrate on their core competencies while ensuring that clinical trials are executed with precision and adherence to legal standards.
This article has highlighted the multifaceted roles of CROs, showcasing their evolution from basic data management to comprehensive clinical trial management. Innovations such as digital data collection and patient engagement strategies illustrate how CROs like bioaccess® not only streamline processes but also markedly reduce timelines for ethical approvals and patient enrollment. The statistics presented, including the fact that CROs conduct nearly 75% of clinical studies globally, underscore their crucial position in advancing medical research and improving patient outcomes.
The significance of CROs in the clinical research ecosystem is paramount. As the industry evolves, embracing technological advancements and globalization, the collaboration between sponsors and CROs will be essential for navigating the complexities of modern clinical trials. This partnership not only accelerates the introduction of innovative therapies into the market but also enhances the overall quality of research data, ultimately leading to improved healthcare solutions. Engaging with CROs represents a strategic move for organizations aiming to excel in the competitive landscape of drug development, ensuring they remain at the forefront of medical innovation.
What are Contract Research Organizations (CROs)?
Contract Research Organizations (CROs) are specialized entities that provide outsourced services to the pharmaceutical, biotechnology, and medical device sectors, managing various elements of clinical studies.
What services do CROs offer in clinical studies?
CROs manage several aspects of clinical studies, including study design, patient recruitment, data management, and compliance with regulations.
How do CROs benefit sponsors in drug development?
By offering vital services, CROs allow sponsors to focus on their primary strengths while ensuring that trials are conducted effectively and in compliance with legal standards.
Can you provide an example of a CRO and its impact?
Bioaccess® is an example of a CRO that accelerates research by leveraging regulatory speed in Latin America, achieving ethical approvals in just 4-6 weeks and enrollment rates that are 50% faster than traditional markets.
What recent advancements have been made in CRO services?
Recent advancements include the implementation of digital data collection technologies and enhanced patient engagement strategies, such as the use of wearables in studies, which improve data precision and simplify procedures.
Why are CROs significant in the pharmaceutical and biotechnology sectors?
CROs play a crucial role in the drug development process, facilitating the transition from research to market and fostering innovation and efficiency within these sectors.