


The article emphasizes the critical classification of medical devices by the FDA into three primary classes based on risk, a fundamental aspect for ensuring safety and regulatory compliance. It delineates that:
Understanding these classifications is essential for manufacturers, enabling them to effectively navigate regulatory pathways.
Understanding the FDA's classification of medical devices is crucial for ensuring patient safety and effective regulatory compliance. By categorizing devices into three distinct classes—Class I, II, and III—the FDA establishes a framework that dictates the level of oversight required based on the associated risks. This article delves into the intricacies of the classification process, highlighting the benefits for manufacturers navigating the complex landscape of medical device regulation. However, with varying requirements and pathways for each class, manufacturers must strategically align their approaches to meet these standards and ensure successful market entry.
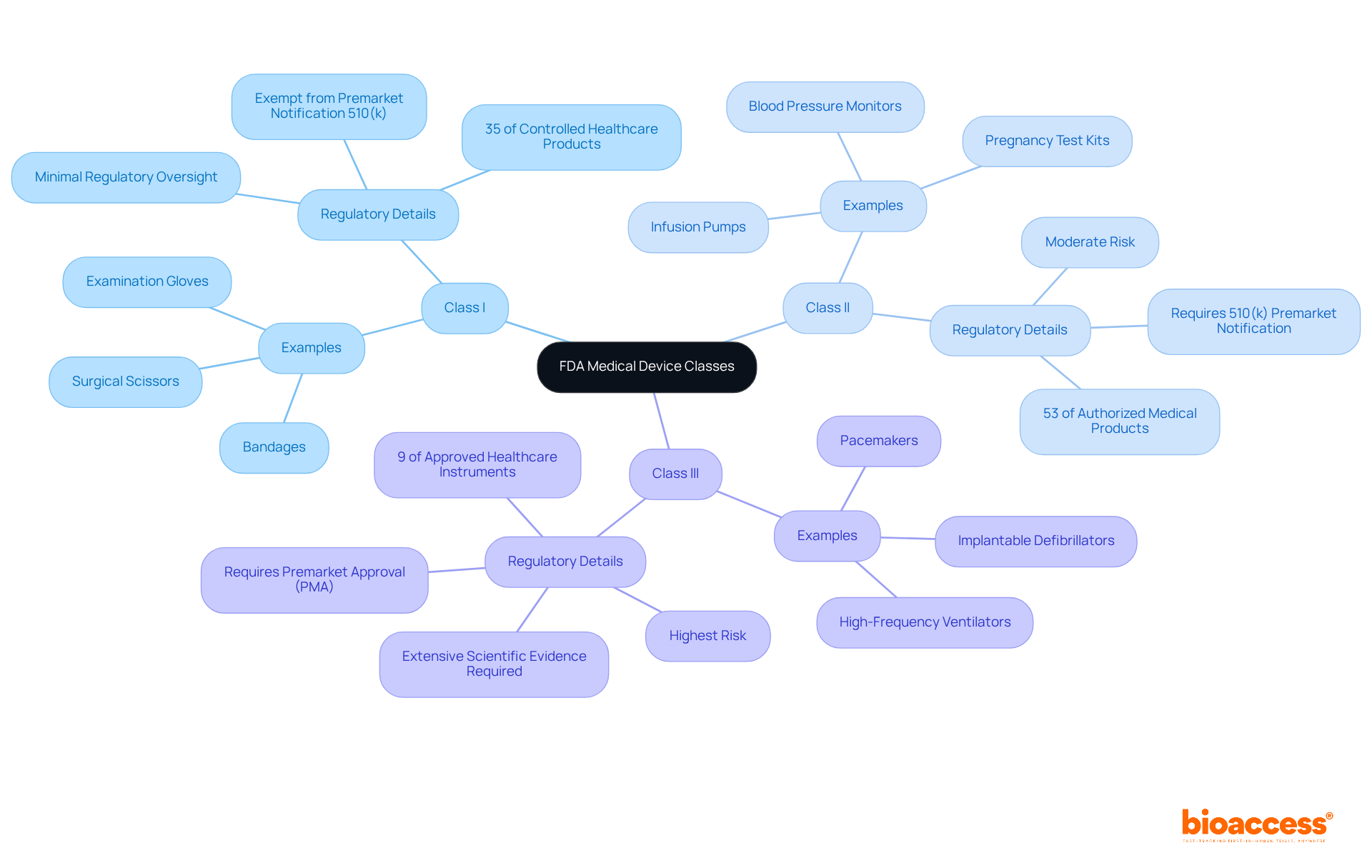
The FDA categorizes medical devices into three primary classes based on their risk to patients and the regulatory controls required to ensure safety and effectiveness:
Class I: Representing the lowest risk, these devices are subject to minimal regulatory oversight. Examples include bandages, surgical scissors, and examination gloves. Notably, around 35% of controlled healthcare products fall into this category, with most Type I items exempt from premarket notification obligations.
Category II: These instruments present a moderate risk and require more rigorous oversight. Common examples include infusion pumps, blood pressure monitors, and pregnancy test kits. Typically, Category II products need a 510(k) premarket notification to demonstrate safety and effectiveness, representing approximately 53% of all authorized medical products.
Category III: This classification includes items associated with the highest risk, subject to the most stringent regulatory oversight. Examples encompass pacemakers, implantable defibrillators, and high-frequency ventilators. Category III products necessitate premarket approval (PMA), which demands significant scientific proof to ensure safety and efficacy. As of 2020, only 9% of approved healthcare instruments are classified as Class III, underscoring the complexity and extensive data requirements involved in their approval process.
In Colombia, the regulatory framework is monitored by INVIMA (Instituto Nacional de Vigilancia de Medicamentos y Alimentos), which plays a crucial role in the inspection and oversight of health products, including healthcare instruments. Established in 1992 under the Ministry of Health and Social Protection, INVIMA is responsible for ensuring compliance with health standards and best practices. The Directorate for Healthcare Instruments and other Technologies within INVIMA oversees and regulates healthcare tools, tracks pre- and post-market initiatives, and recommends technical standards for production and quality assurance. The organization is recognized as a Level 4 health authority by the Pan American Health Organization/World Health Organization, signifying its proficiency in overseeing the safety, efficacy, and quality of healthcare products. Understanding both the FDA class system and INVIMA's regulatory framework is essential for manufacturers to navigate the complexities of compliance in different markets effectively.
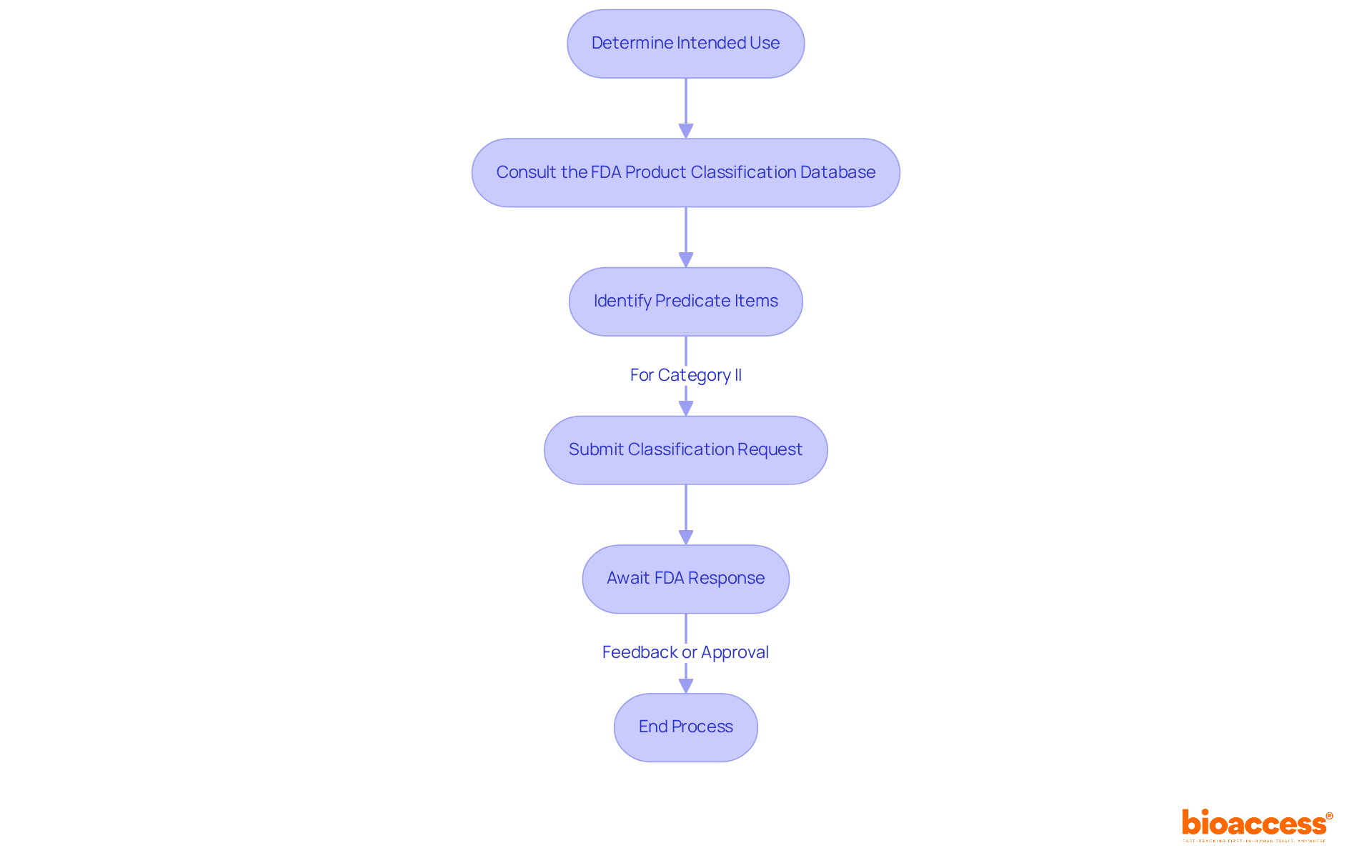
The classification process for medical devices is a critical endeavor that involves several essential steps:
By following these steps, manufacturers can effectively categorize their products and ensure adherence to the FDA class regulations, ultimately facilitating a smoother path to market. Furthermore, pursuing specialized assistance, such as from professionals like Ana Criado, who possesses extensive experience in compliance matters and biomedical engineering, can aid in navigating the complexities of the classification process. Moreover, Katherine Ruiz's knowledge in compliance matters for healthcare instruments and in vitro diagnostics in Colombia can offer further insights into the oversight environment, ensuring a thorough grasp of the classification procedure.
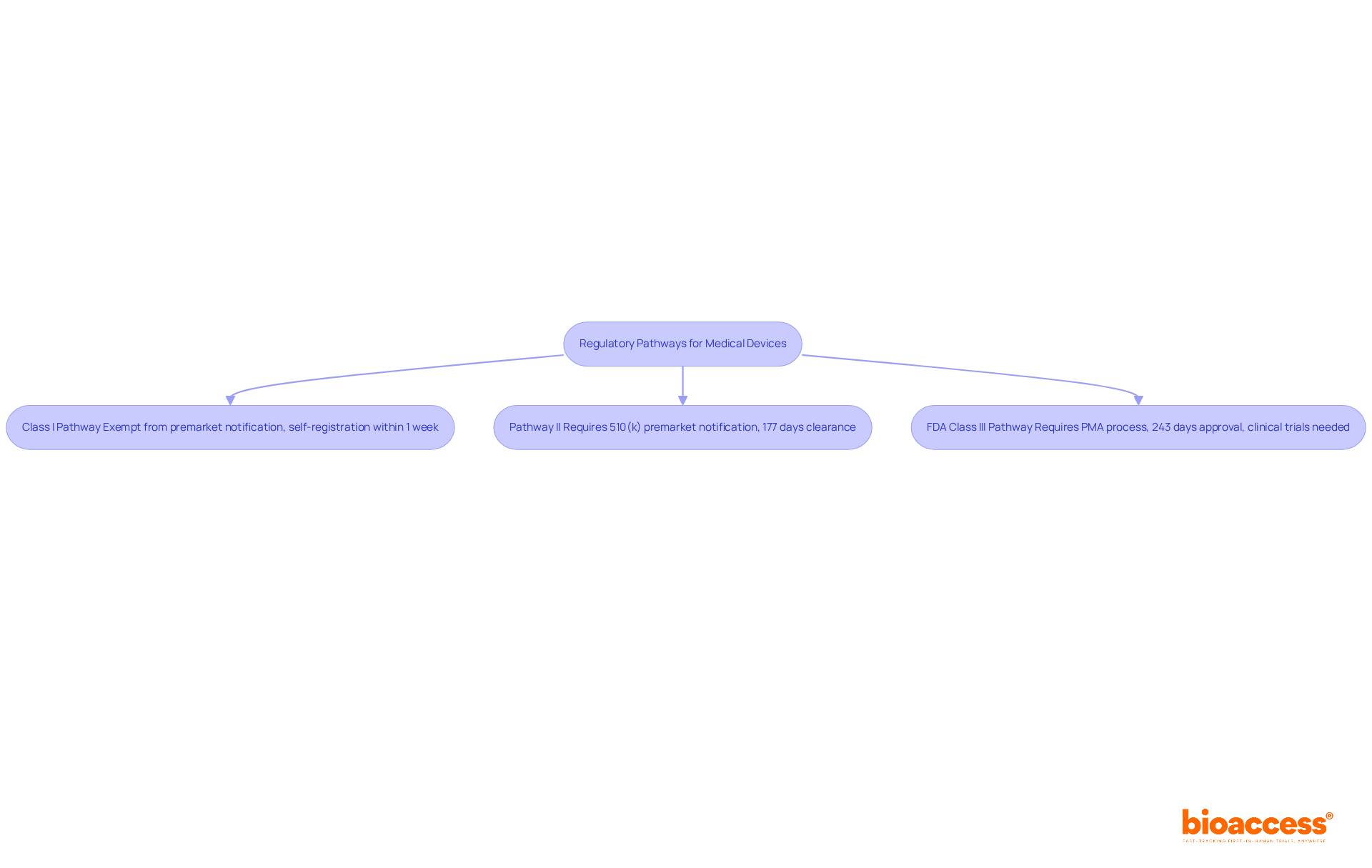
The regulatory pathways for medical devices are determined by their classification, which significantly influences the approval process:
Class I Pathway: Most Class I devices are exempt from premarket notification, allowing for a streamlined entry into the market. However, manufacturers must still adhere to general controls, including registration and compliance with quality system regulations. This pathway typically allows for rapid market access, often completing the self-registration process within about one week.
Pathway II: Pathway II products typically need a 510(k) premarket notification, which falls under the FDA class regulations that require showing substantial equivalence to an already legally marketed product. Manufacturers must submit data on safety and effectiveness, along with comprehensive labeling information. The typical clearance duration for Category II items is roughly 177 days, though this can fluctuate depending on the type of item and submission quality. Notably, 85 percent of 510(k) applications received a Substantially Equivalent decision in recent evaluations, indicating a favorable approval landscape.
FDA Class III Pathway: FDA Class products, specifically Class III, include high-risk items such as cochlear implants and defibrillators, which undergo a more stringent premarket approval (PMA) process. This pathway requires extensive clinical data to demonstrate safety and efficacy, often necessitating clinical trials. The average PMA application is currently approved in about 243 days, reflecting improvements in the approval timeline while maintaining rigorous safety standards.
Understanding these regulatory pathways is essential for manufacturers aiming to navigate the complexities of the medical device landscape effectively. By aligning their strategies with the specific requirements of each class, companies can enhance their chances of timely market entry and compliance.
The classification of medical devices by the FDA represents a vital framework that guarantees patient safety and product efficacy. By categorizing devices into three distinct classes according to their associated risks, manufacturers obtain a clearer understanding of the regulatory requirements necessary to bring their products to market. This structured approach not only facilitates compliance but also enhances the overall safety of medical devices available to consumers.
Throughout the article, the distinctions between Class I, Class II, and Class III devices have been thoroughly examined. Class I devices, characterized by minimal regulatory oversight, enable quick market access, whereas Class II devices require a more detailed submission process to demonstrate safety and effectiveness. In contrast, Class III devices encounter the most stringent requirements, often necessitating extensive clinical trials and substantial data to secure approval. Moreover, the role of INVIMA in Colombia underscores the significance of understanding regional regulatory frameworks alongside the FDA's guidelines.
In conclusion, navigating the complexities of medical device classification is imperative for manufacturers seeking to ensure compliance and promote patient safety. By familiarizing themselves with the classification process and regulatory pathways, manufacturers can streamline their product development and approval strategies. Collaborating with experts in the field can further enhance understanding and execution of these processes, ultimately contributing to safer healthcare outcomes.
What are the three classes of medical devices defined by the FDA?
The FDA categorizes medical devices into three classes: Class I (low risk), Class II (moderate risk), and Class III (high risk).
What are the characteristics of Class I medical devices?
Class I devices represent the lowest risk and are subject to minimal regulatory oversight. Examples include bandages, surgical scissors, and examination gloves. Most Class I items are exempt from premarket notification obligations.
What is required for Class II medical devices?
Class II devices present a moderate risk and require more rigorous oversight. They typically need a 510(k) premarket notification to demonstrate safety and effectiveness. Common examples include infusion pumps and blood pressure monitors.
How are Class III medical devices regulated?
Class III devices are associated with the highest risk and are subject to the most stringent regulatory oversight. They require premarket approval (PMA), which demands significant scientific evidence to ensure safety and efficacy. Examples include pacemakers and implantable defibrillators.
What percentage of medical devices fall into each FDA class?
Approximately 35% of controlled healthcare products are Class I, about 53% are Class II, and only 9% are classified as Class III.
What role does INVIMA play in Colombia's medical device regulation?
INVIMA (Instituto Nacional de Vigilancia de Medicamentos y Alimentos) monitors and regulates health products in Colombia, ensuring compliance with health standards. It oversees healthcare instruments, tracks pre- and post-market initiatives, and recommends technical standards for production and quality assurance.
What is the significance of INVIMA's recognition by the Pan American Health Organization/World Health Organization?
INVIMA is recognized as a Level 4 health authority, indicating its proficiency in overseeing the safety, efficacy, and quality of healthcare products.
Why is it important for manufacturers to understand both the FDA class system and INVIMA's regulatory framework?
Understanding both regulatory frameworks is essential for manufacturers to effectively navigate compliance complexities in different markets.