


This article provides a comprehensive overview of the FDA's classification of medical devices into three distinct classes, a process that is crucial for ensuring compliance and safety in clinical research.
The accurate classification of these devices is paramount, as it directly impacts regulatory compliance, market access, and ultimately, patient safety.
Understanding the classification of medical devices is essential for navigating the complex regulatory landscape that governs their safety and efficacy. The FDA's system categorizes devices into three distinct classes, each with varying levels of risk and regulatory requirements, thereby influencing market access and compliance strategies. However, the challenge lies in accurately determining the appropriate classification, as missteps can lead to serious consequences, including financial loss and compromised patient safety.
How can manufacturers ensure they are correctly classifying their products to meet both regulatory standards and market demands?
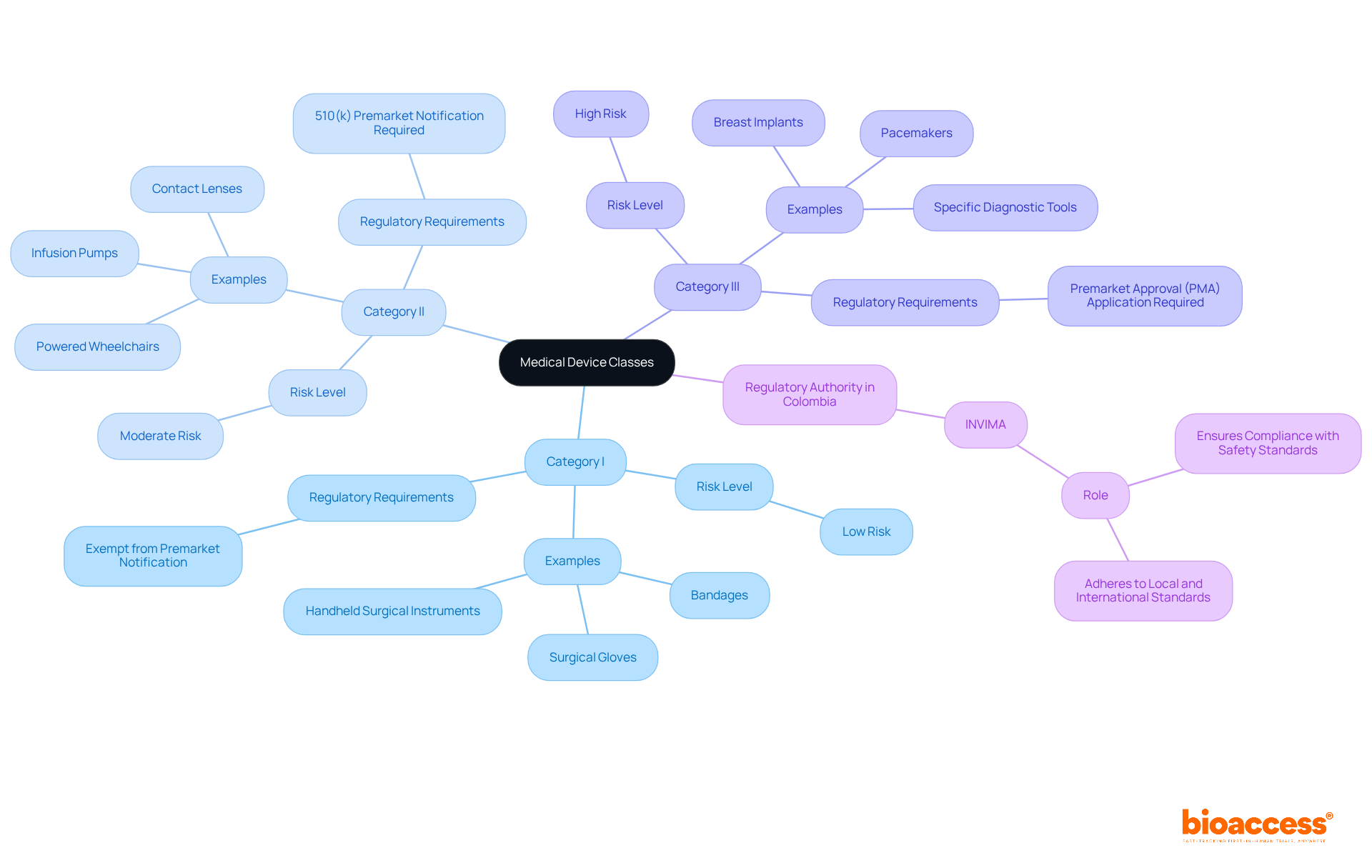
The FDA divides medical instruments into three groups referred to as FDA medical device classes, based on their intended use and the level of risk they pose to patients. This classification is crucial for determining the regulatory controls necessary to ensure safety and effectiveness. A medical instrument encompasses any tool, apparatus, implement, machine, contrivance, implant, in vitro reagent, or similar article intended for use in the diagnosis, cure, mitigation, treatment, or prevention of disease. Understanding this definition is the first step in navigating the regulatory landscape for medical equipment.
In Colombia, the National Food and Drug Surveillance Institute (INVIMA) serves a pivotal role akin to that of the FDA in the United States. Established in 1992 under the Ministry of Health and Social Protection, this organization is tasked with inspecting and supervising the marketing and manufacturing of health products, including medical instruments. It ensures compliance with health standards and implements best practices for the import and export of these items. The Directorate for Medical Equipment and other Technologies within INVIMA oversees and regulates medical instruments, proposing technical standards for their production, promotion, and quality assurance.
The classification system for FDA medical device classes categorizes items into:
Each group has distinct requirements for premarket submission and regulatory controls. Notably, INVIMA has been designated as a Level 4 regional reference health authority by the Pan American Health Organization/World Health Organization, reflecting its capability and effectiveness in health regulation. This classification is essential for ensuring the safety, efficacy, and quality of medical products in Colombia.
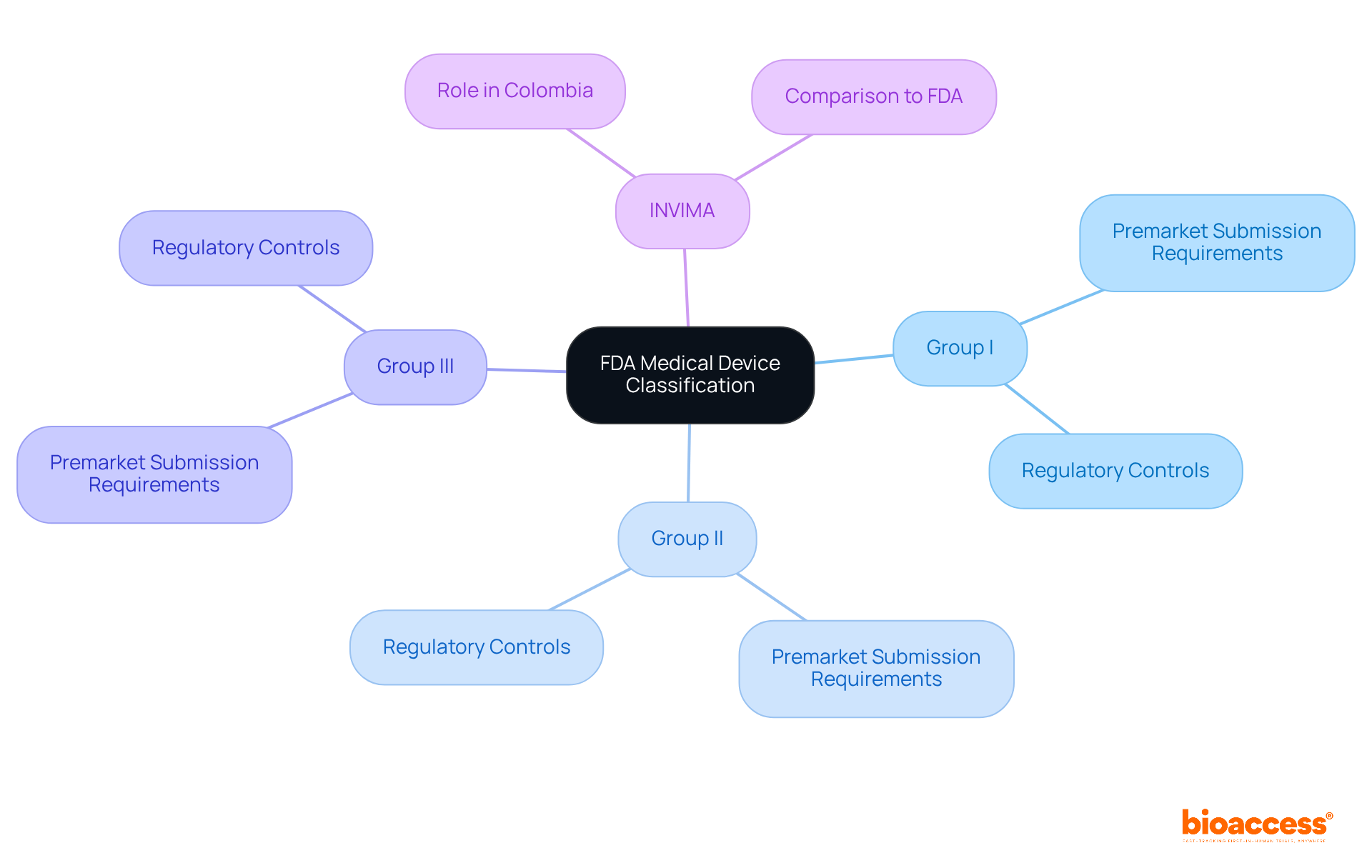
Category I items are classified as low-risk products, subject to minimal regulatory oversight. Examples include bandages, surgical gloves, and handheld surgical instruments. Most products classified under FDA medical device classes, specifically Category I, are exempt from premarket notification obligations, indicating that they do not require FDA approval prior to marketing.
Category II equipment presents moderate risk and necessitates increased regulatory oversight to ensure safety and efficacy. Examples include infusion pumps, contact lenses, and powered wheelchairs. Category II products, as classified under the FDA medical device classes, generally require a 510(k) premarket notification to demonstrate that the item is substantially equivalent to a legally marketed product.
Category III equipment comprises high-risk items that demand the most rigorous regulatory oversight. Typically intended to support or sustain life, these products also pose a potential unreasonable risk of illness or injury. Examples include pacemakers, breast implants, and specific diagnostic tools. Class III products, which fall under the FDA medical device classes, necessitate a Premarket Approval (PMA) application, involving a thorough review process to guarantee safety and effectiveness.
In Colombia, the regulation of medical instruments falls under the authority of the National Institute for the Surveillance of Medicines and Foods. This organization is tasked with ensuring that health products comply with safety and effectiveness standards. As a Level 4 health authority recognized by the Pan American Health Organization/World Health Organization, INVIMA plays a crucial role in regulating medical equipment, ensuring adherence to both local and international standards.
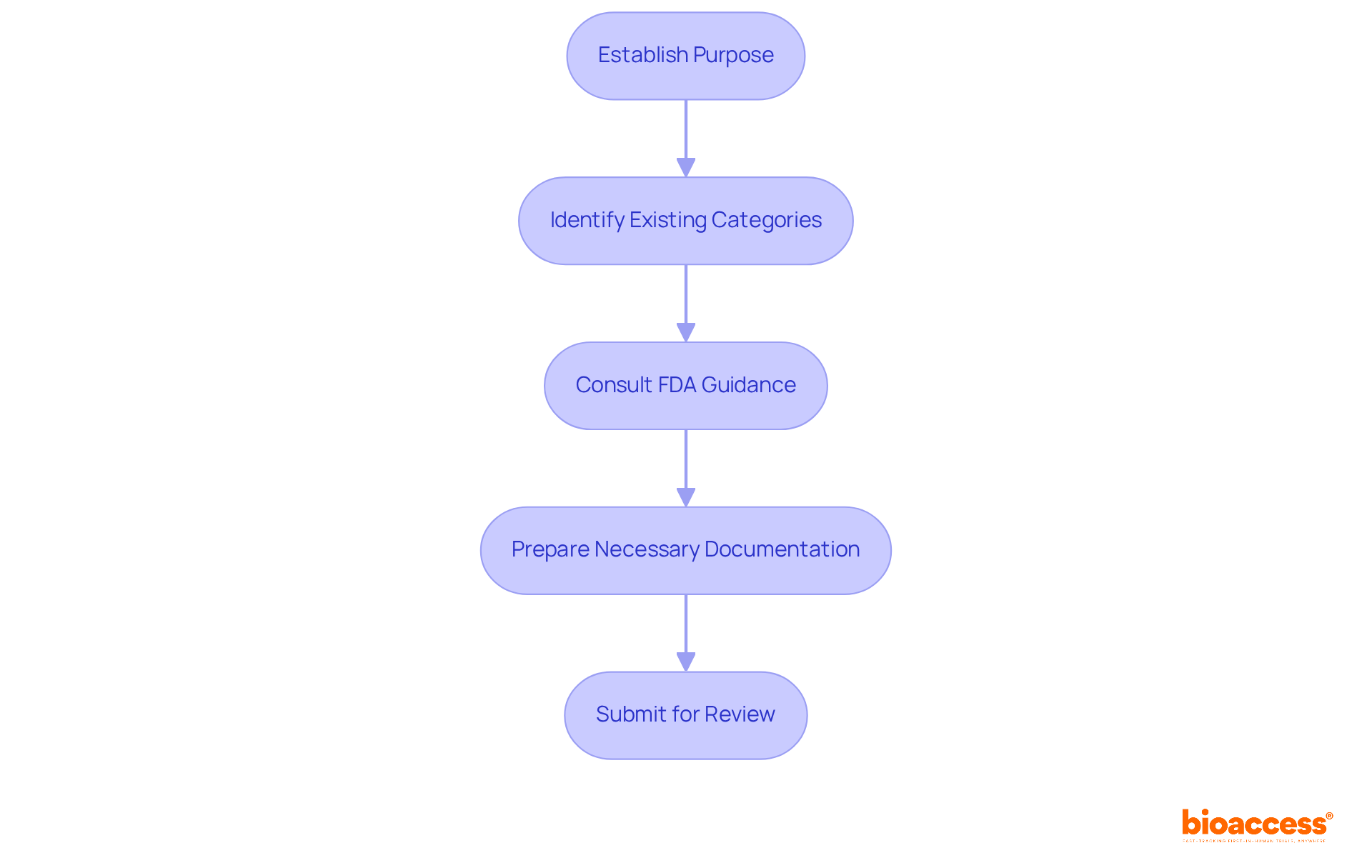
Establish Purpose: The initial phase in the categorization process is to clearly specify the intended purpose of the apparatus. This encompasses grasping the assertions that will be presented regarding the apparatus and its intended patient group.
Identify Existing Categories: Utilize the FDA's categorization database to find existing categories that align with your product's intended use, specifically within the FDA medical device classes. This can provide a reference point for determining the appropriate class.
Consult FDA Guidance: Review FDA guidance documents pertaining to your product type. These documents frequently offer valuable perspectives on the sorting process and any particular requirements that may be relevant.
Prepare Necessary Documentation: Depending on the class, prepare the required documentation for submission. For items categorized under the FDA medical device classes, Class I may require minimal data, while Class II and III products will necessitate more comprehensive data, including clinical studies for Class III.
Submit for Review: Send your categorization request or premarket notification to the FDA. Be prepared to respond to any questions or requests for additional information from the FDA during the review process.
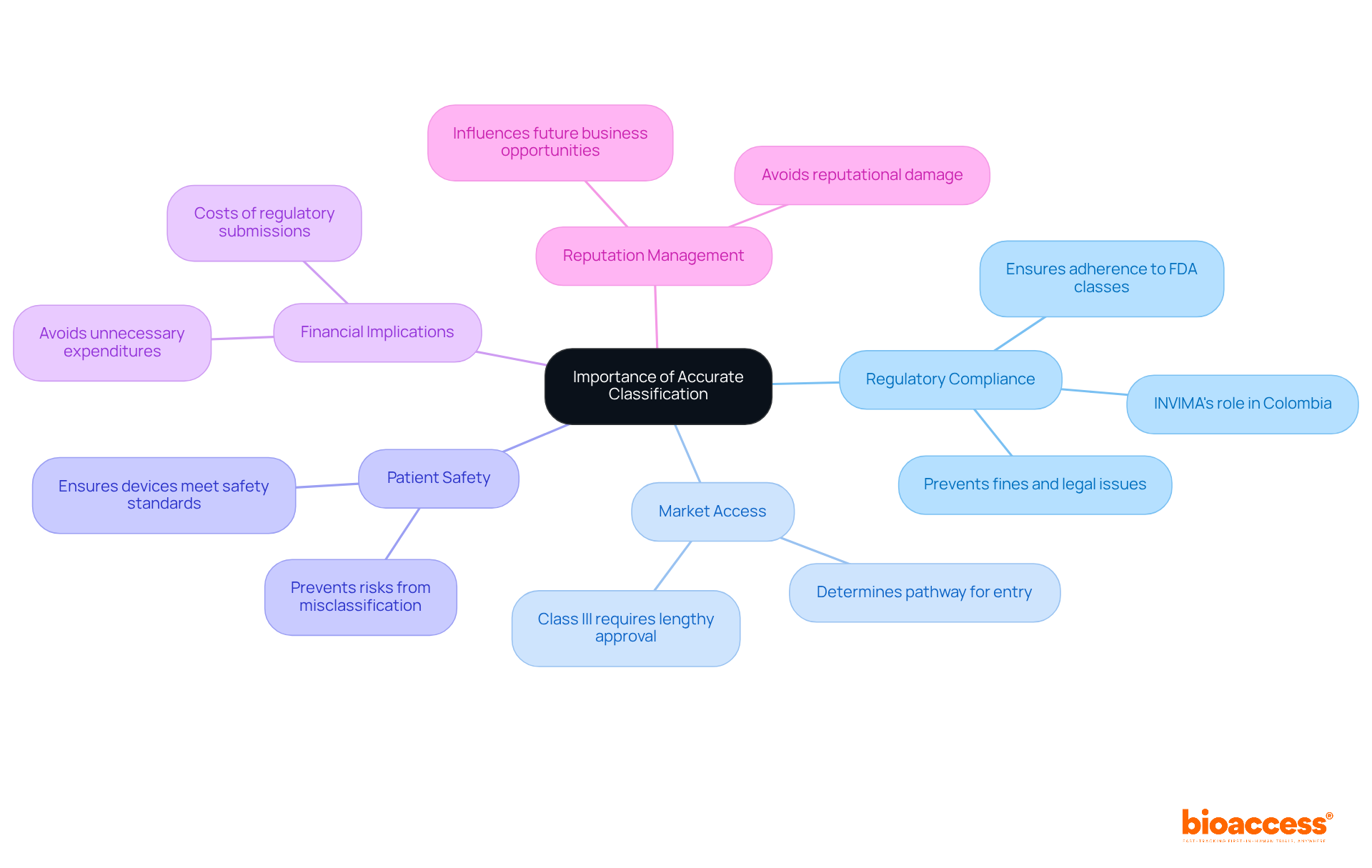
Accurate classification of medical devices is paramount for several reasons:
Regulatory compliance is essential because misclassifying a device can lead to non-compliance with FDA medical device classes, which may result in fines, product recalls, or even legal action. In Colombia, the INVIMA (Colombia National Food and Drug Surveillance Institute) plays a crucial role in ensuring compliance with health regulations. It oversees the marketing and manufacturing of health products and provides medical approval for imports and exports.
Market Access: The classification determines the pathway for market entry. Class III products, for instance, necessitate a more comprehensive and lengthy approval procedure compared to Class I and II items. The regulatory authority's oversight ensures that devices meet the necessary standards for market access in Colombia.
Patient Safety: Incorrect categorization can jeopardize patient safety. Devices that are not adequately regulated may pose risks to users, leading to adverse health outcomes. The agency's categorization and oversight procedures are intended to protect patient health by implementing stringent regulatory standards.
Financial Implications: The costs associated with regulatory submissions can be significant. Misclassification can lead to unnecessary expenditures and delays in product launch, impacting a company's bottom line. Understanding the regulatory framework of the FDA medical device classes can help companies efficiently manage these financial challenges.
Reputation Management: Companies that face regulatory issues due to misclassification may suffer reputational damage, affecting future business opportunities and partnerships. As a Level 4 health authority recognized by PAHO/WHO, INVIMA's credibility adds an additional layer of importance to compliance and classification efforts in the region.
Understanding the classification of medical devices by the FDA is essential for ensuring compliance and maintaining safety standards in healthcare. This article outlines the three distinct classes of medical devices—Class I, Class II, and Class III—each with varying levels of risk and regulatory requirements. This classification system guides manufacturers in the development and marketing of their products and plays a pivotal role in safeguarding patient health.
Key aspects of each device class are highlighted, emphasizing the need for careful categorization based on risk levels and intended use.
Furthermore, the importance of regulatory compliance, market access, patient safety, and financial implications is underscored, illustrating the far-reaching consequences of accurate classification.
Navigating the FDA medical device classification process is not merely a bureaucratic step; it is a critical factor that influences patient safety and the successful market entry of medical devices. Manufacturers and stakeholders must prioritize understanding and adhering to these classifications to avoid potential pitfalls, including regulatory penalties and compromised patient safety. By fostering a culture of compliance and diligence, the healthcare industry can ensure that medical devices meet the necessary standards, ultimately contributing to better health outcomes for all.
What are the FDA medical device classes?
The FDA classifies medical devices into three groups based on their intended use and the level of risk they pose to patients, known as FDA medical device classes.
Why is the classification of medical devices important?
The classification is crucial for determining the regulatory controls necessary to ensure the safety and effectiveness of medical devices.
What is considered a medical instrument?
A medical instrument includes any tool, apparatus, implement, machine, contrivance, implant, in vitro reagent, or similar article intended for use in the diagnosis, cure, mitigation, treatment, or prevention of disease.
What role does INVIMA play in Colombia regarding medical devices?
INVIMA, the National Food and Drug Surveillance Institute in Colombia, inspects and supervises the marketing and manufacturing of health products, including medical instruments, ensuring compliance with health standards.
When was INVIMA established and under which ministry?
INVIMA was established in 1992 under the Ministry of Health and Social Protection in Colombia.
What is the function of the Directorate for Medical Equipment and other Technologies within INVIMA?
This directorate oversees and regulates medical instruments, proposing technical standards for their production, promotion, and quality assurance.
How many groups are there in the FDA medical device classification system?
There are three groups in the FDA medical device classification system: Group I, Group II, and Group III.
What is the significance of INVIMA being designated as a Level 4 regional reference health authority?
This designation reflects INVIMA's capability and effectiveness in health regulation, ensuring the safety, efficacy, and quality of medical products in Colombia.