


The article elucidates that 'mabs' denotes monoclonal antibodies—engineered molecules meticulously designed to target specific antigens. These antibodies play a pivotal role in both diagnostics and therapeutics, particularly in the realms of cancer treatment and autoimmune diseases.
The historical evolution of monoclonal antibodies, tracing back to their inception in 1975, highlights significant advancements in production methods and their escalating importance in diverse therapeutic applications. This underscores their transformative impact on modern medicine, establishing a compelling case for their continued development and utilization.
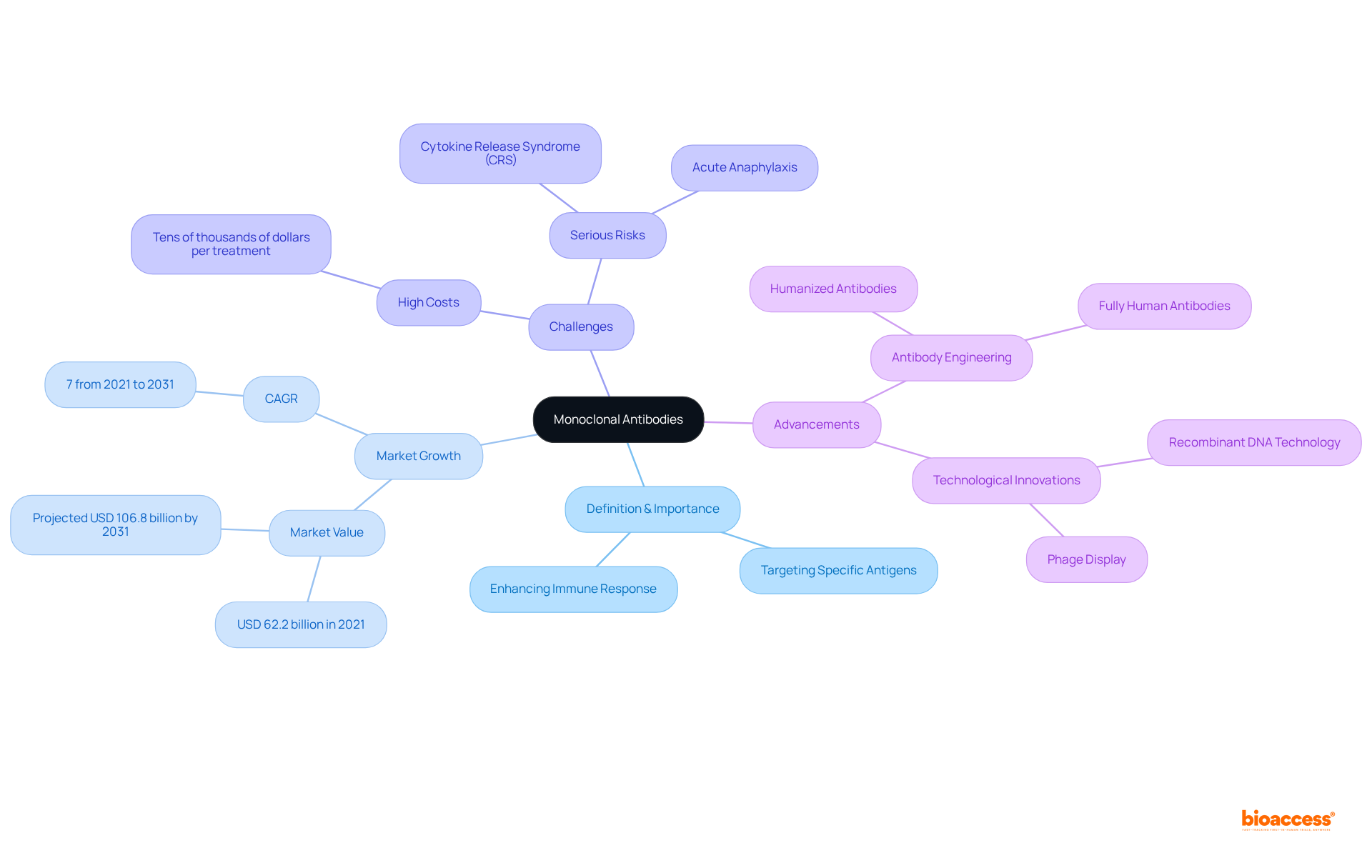
Monoclonal antibodies have revolutionized modern medicine, providing targeted therapies that significantly enhance the immune system's capacity to fight diseases, particularly cancer and autoimmune disorders. As these engineered molecules gain prominence, it is essential to understand their:
to appreciate their pivotal role in healthcare. However, with these advancements come challenges, such as high production costs and potential adverse effects, which raise critical questions regarding accessibility and safety.
What does the future hold for monoclonal antibodies? How can their potential be fully realized in the pursuit of effective treatments?
Monoclonal antibodies are laboratory-created molecules designed to serve as replacements for natural antibodies. They are specifically intended to attach to particular antigens, typically proteins found on the surface of various units, including cancerous cells. The precision of monoclonal antibodies allows them to target specific cells or pathogens, making them indispensable in diagnostics and therapeutics. Their significance lies in their capacity to enhance the immune response against diseases, particularly in oncology, autoimmune disorders, and infectious diseases. The mabs meaning, which involves mimicking the immune system's ability to combat harmful invaders, has revolutionized treatment protocols and markedly improved patient outcomes.
The global cancer monoclonal proteins market was valued at USD 62.2 billion in 2021 and is projected to reach USD 106.8 billion by 2031, indicating a robust growth trajectory. Mukesh Kumar from Thomas Jefferson University remarked, "The potential of these molecules is reflected in the market value of the monoclonal proteins in cancer treatment, which is anticipated to reach approximately USD 159.96 billion by 2030 from USD 62.2 billion in 2021." However, the high costs associated with monoclonal therapies present challenges for broader adoption, particularly in low- and middle-income nations. Furthermore, serious risks such as acute anaphylaxis and cytokine release syndrome (CRS) are linked to mAb treatments, underscoring the necessity for careful patient management.
Advancements in antibody engineering methods have also been crucial in the evolution and effectiveness of monoclonal proteins. This progress has led to the development of humanized and fully human substances that exhibit decreased immunogenicity and enhanced therapeutic efficacy.
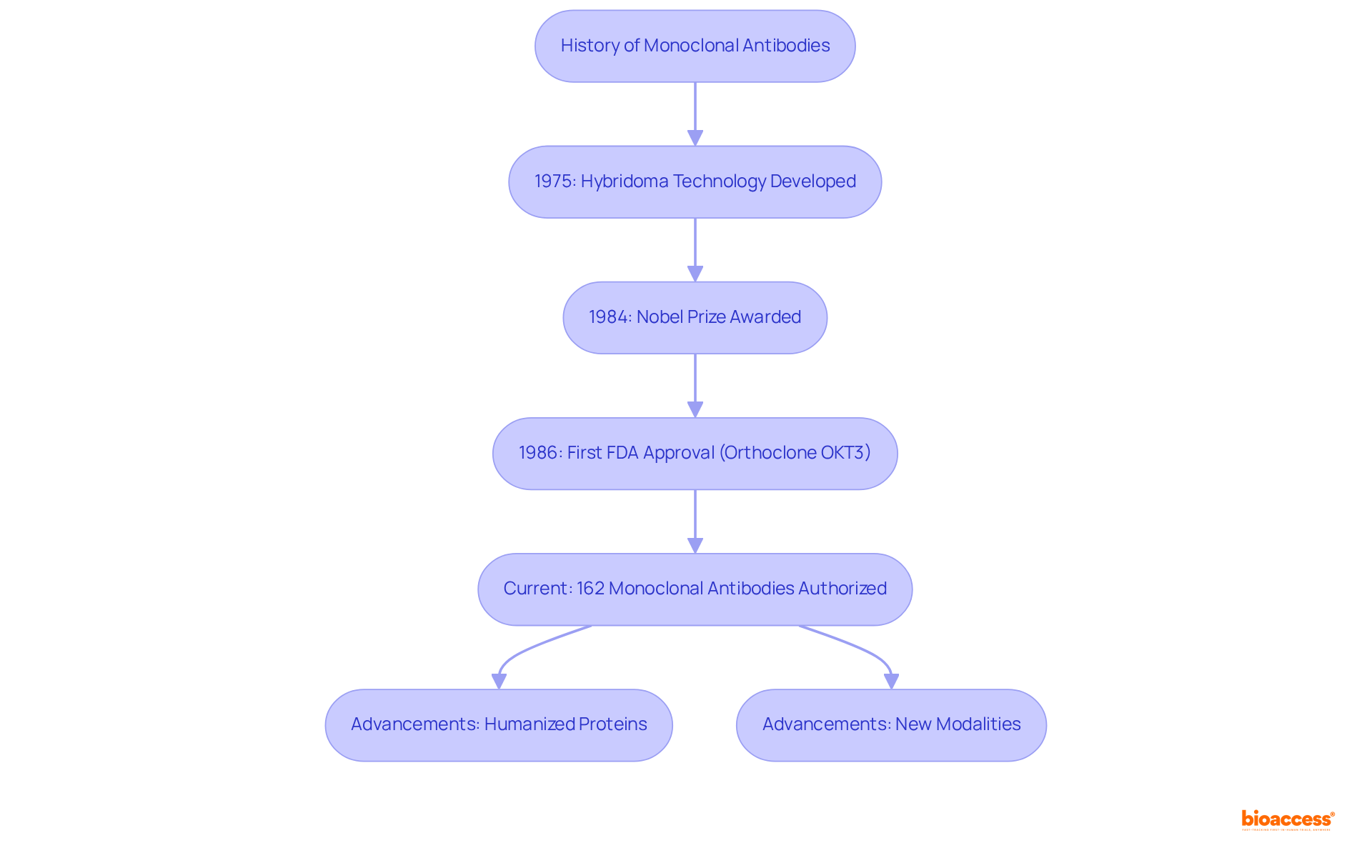
The journey of monoclonal antibodies began in 1975 when Georges Köhler and César Milstein pioneered hybridoma technology, which enabled the generation of identical antibodies from a single clone of B cells. This groundbreaking achievement earned them the Nobel Prize in Physiology or Medicine in 1984.
The first fully licensed monoclonal antibody, Orthoclone OKT3, received FDA approval in 1986 for preventing kidney transplant rejection, marking a significant milestone in medical history. Since then, the field has experienced rapid growth, with 162 monoclonal antibodies currently authorized for various treatment applications, including cancer care, autoimmune conditions, and infectious illnesses.
The advancement of monoclonal antibodies has been characterized by innovations in engineering methods, resulting in the development of humanized and entirely human proteins that reduce immunogenicity and enhance clinical effectiveness. Notably, monoclonal proteins now account for nearly 20% of the FDA's new medication approvals annually, a figure that has remained steady over the years, underscoring their essential role in contemporary therapeutics.
Moreover, advancements in immune protein modalities, such as drug-linked conjugates (ADCs) and bispecific immune proteins, continue to shape the landscape of monoclonal antibody development.
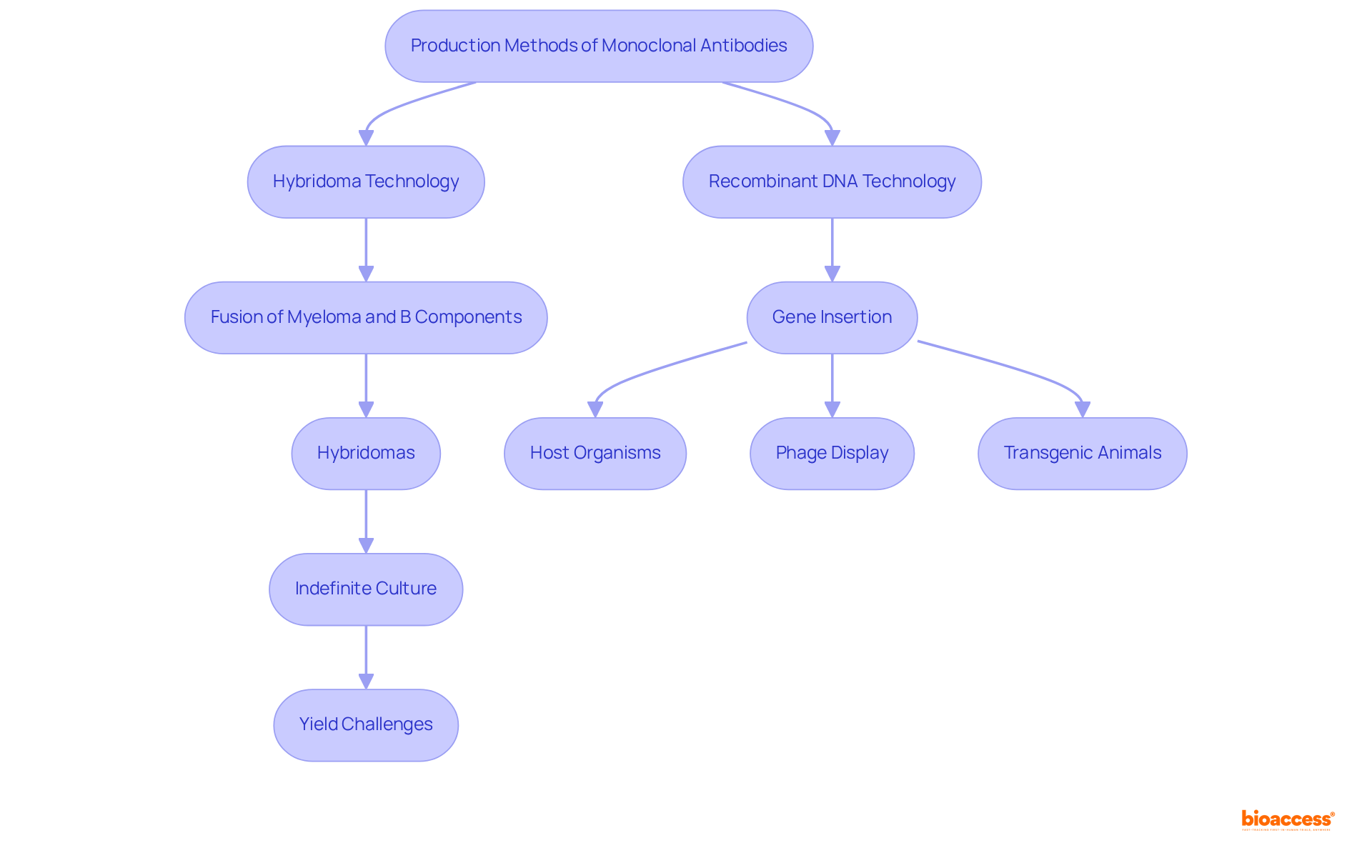
The term 'mAbs meaning' refers to monoclonal antibodies, which are primarily generated through hybridoma technology, a process that involves merging myeloma components with B components responsible for producing the desired immunoglobulin. This fusion results in hybrid entities, known as hybridomas, which are capable of indefinite culture and large-scale immunoglobulin production. A critical aspect of this process is the immortalization of splenocytes via somatic fusion with a myeloma line partner, essential for sustaining hybridoma growth. However, the growth and yield of hybridomas can be unpredictable, often influenced by feeder layers and exhibiting genetic instability, which complicates scalability.
In contrast, recombinant DNA technology has emerged as a powerful alternative, facilitating the insertion of gene sequences that encode immunoglobulins into host organisms, such as bacteria or yeast, to enhance immunoglobulin synthesis. Recent advancements, including phage display and the utilization of transgenic animals, have markedly improved the efficiency and specificity of mAb production. Notably, only 1 out of over 100 FDA-approved therapeutic proteins is currently produced using hybridoma technology, underscoring the shift towards recombinant production due to its superior control over yield and purity.
As we look towards 2025, advancements in hybridoma technology continue to progress, addressing some of its inherent limitations. For example, stable cell lines now differentiate immunoglobulin selection from expression vehicle selection, enabling better control over production processes. This transition is vital as the demand for high-quality monoclonal proteins in clinical applications continues to rise. The integration of recombinant DNA technology not only enhances the scalability of mAb production but also helps clarify mAbs meaning by ensuring that the proteins generated meet high purity standards, essential for medical applications. CHO lines, commonly employed in recombinant immune protein production, provide traceable origins and reliability, further emphasizing the advantages of this approach.
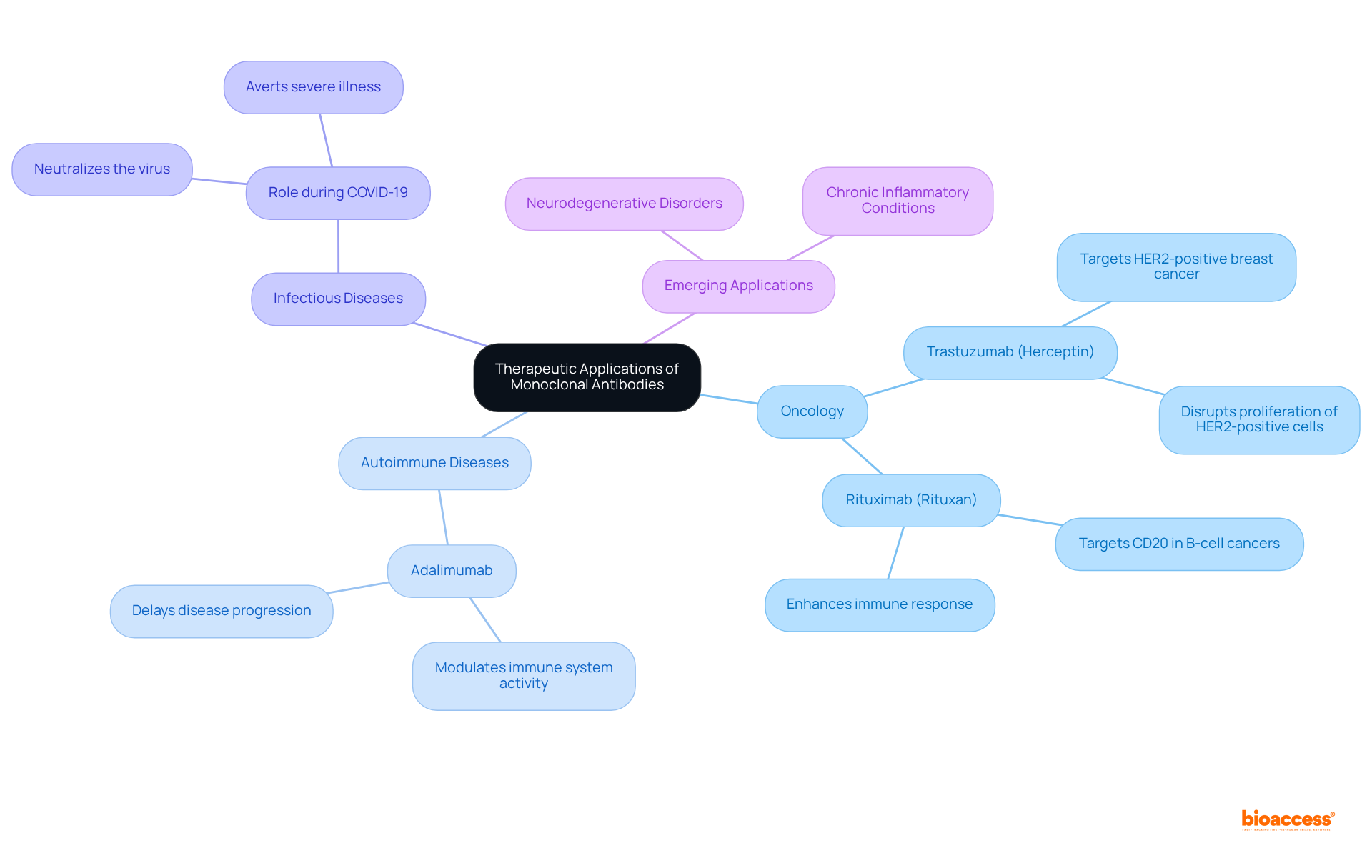
Monoclonal antibodies have emerged as crucial therapeutic agents, particularly in oncology, where they specifically target cancerous tissues to enhance immune responses and impede tumor growth. A notable example is trastuzumab (Herceptin), a well-recognized mAb utilized in treating HER2-positive breast cancer. It effectively disrupts the proliferation of HER2-positive cells while minimizing damage to healthy tissue. Similarly, rituximab (Rituxan) targets CD20 in B-cell cancers, exemplifying the precision of monoclonal antibodies in cancer therapy.
Beyond oncology, monoclonal antibodies play a vital role in managing autoimmune diseases such as rheumatoid arthritis and multiple sclerosis. They modulate the immune system's activity, offering effective treatment options that can delay disease progression. The success rates of monoclonal antibodies in these conditions are remarkable, with therapies like adalimumab demonstrating significant improvements in patient outcomes.
In the realm of infectious diseases, monoclonal antibodies have gained prominence, especially during the COVID-19 pandemic, where they are employed to neutralize the virus and avert severe illness. Interim results from a recent trial involving DNA-encoded monoclonal antibodies (DMAbs) revealed that 100% of participants maintained stable DMAb levels for 72 weeks, suggesting potential for long-term therapeutic solutions. Furthermore, the effectiveness of clinical trials featuring monoclonal antibodies is underscored by the fact that enrollment occurs 50% more swiftly than in conventional markets, thereby enhancing patient access to these treatments.
The versatility of monoclonal antibodies continues to expand, with ongoing studies exploring their applications in neurodegenerative disorders and chronic inflammatory conditions. Nevertheless, challenges such as manufacturing complexity and high costs remain significant considerations in the development and deployment of therapies, which relate to mAbs meaning. As the biopharmaceutical market evolves, monoclonal antibodies remain at the forefront of precision medicine, providing targeted, effective, and personalized treatments across various medical domains.
Monoclonal antibodies (mAbs) signify a monumental leap in medical science, delivering targeted therapies that emulate the immune system's inherent capacity to combat diseases. Their precision in identifying and binding to specific antigens has revolutionized treatment protocols across various fields, particularly in oncology, autoimmune disorders, and infectious diseases. Understanding the meaning of mAbs encompasses not only their definition but also their historical evolution and extensive applications, highlighting their vital role in contemporary medicine.
This article has explored key concepts such as the history of monoclonal antibodies, their production methods, and therapeutic applications. From the groundbreaking work of Köhler and Milstein in 1975 to the latest advancements in antibody engineering, the evolution of mAbs illustrates their escalating significance and the relentless innovation within this domain. Although challenges such as high costs and complex manufacturing processes persist, the impressive success rates in treating conditions like cancer and autoimmune diseases underscore their potential.
As research advances, the future of monoclonal antibody therapy appears promising, with ongoing investigations into new applications and production techniques. The importance of monoclonal antibodies in precision medicine cannot be overstated; they provide hope for more effective and personalized treatments. Engaging with the advancements and challenges in this area is crucial for healthcare professionals and patients alike, as the journey of mAbs continues to redefine the landscape of therapeutic interventions.
What are monoclonal antibodies?
Monoclonal antibodies are laboratory-created molecules designed to replace natural antibodies, specifically targeting particular antigens, typically proteins found on the surface of cells, including cancerous cells.
Why are monoclonal antibodies important?
They are important because they enhance the immune response against diseases, particularly in oncology, autoimmune disorders, and infectious diseases, leading to improved patient outcomes.
What is the significance of the monoclonal antibodies market?
The global cancer monoclonal proteins market was valued at USD 62.2 billion in 2021 and is projected to reach USD 106.8 billion by 2031, indicating robust growth and the potential for these molecules in cancer treatment.
What challenges are associated with monoclonal antibody therapies?
High costs associated with monoclonal therapies present challenges for broader adoption, especially in low- and middle-income nations. Additionally, there are serious risks linked to treatments, such as acute anaphylaxis and cytokine release syndrome (CRS).
How have advancements in antibody engineering impacted monoclonal antibodies?
Advancements in antibody engineering have led to the development of humanized and fully human antibodies that exhibit decreased immunogenicity and enhanced therapeutic efficacy, improving their effectiveness in treatment protocols.