


The landscape of clinical research is undergoing a significant transformation, with transparency emerging as a non-negotiable standard in the pursuit of scientific integrity. In Bulgaria, the national clinical trials data transparency rules serve as a pivotal framework designed to ensure that critical research information is accessible to all stakeholders, from researchers to patients. This commitment to openness not only enhances public trust in medical interventions but also raises essential questions about the effectiveness of these regulations in safeguarding patient safety and promoting ethical research practices.
What challenges lie ahead in the implementation of these rules? How will they shape the future of clinical trials in Bulgaria? These questions are crucial as we navigate the evolving Medtech landscape, where bioaccess plays a vital role in addressing key challenges. The importance of collaboration among stakeholders cannot be overstated, as it is essential for fostering an environment that prioritizes patient safety and ethical standards in research.
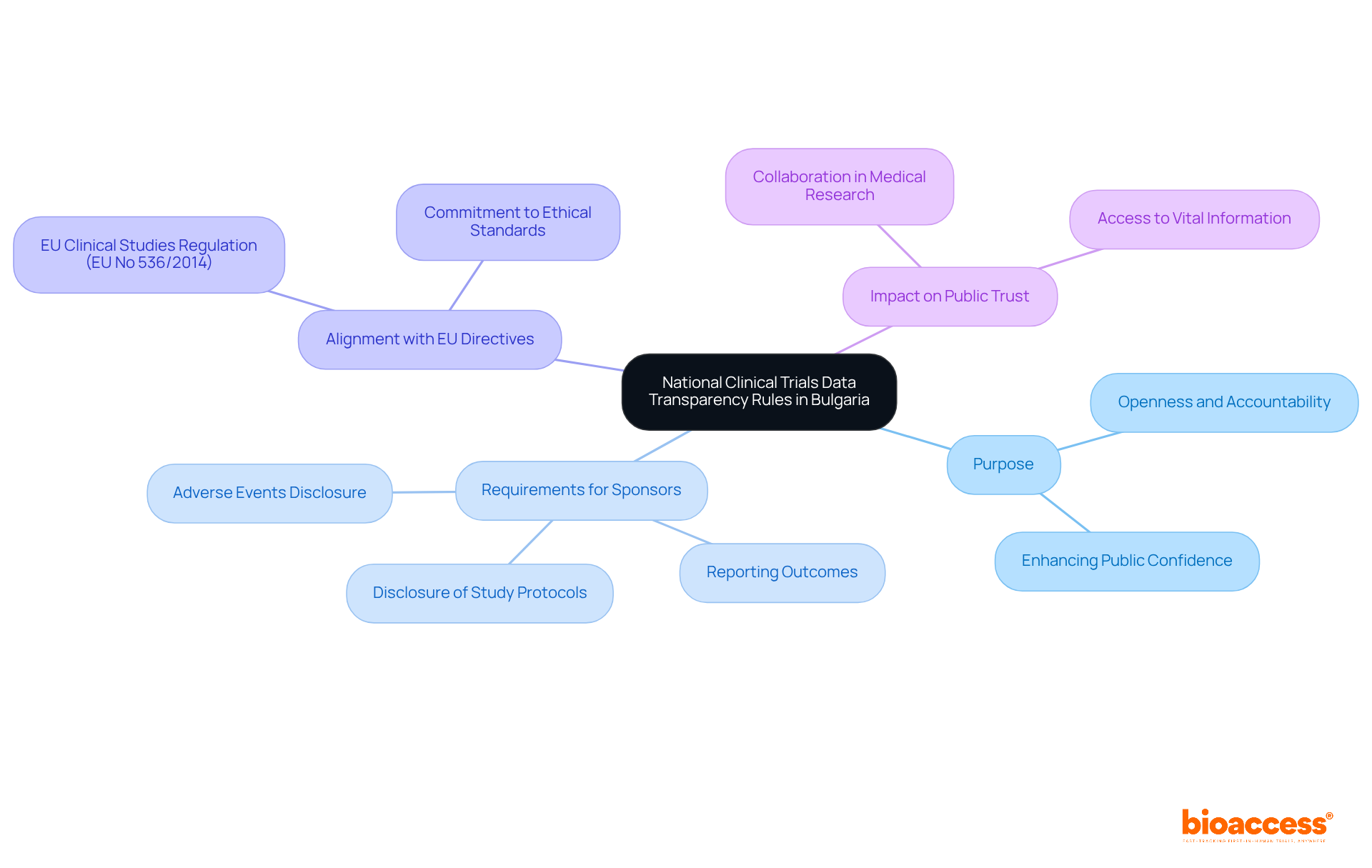
The national clinical trials data transparency rules in Bulgaria represent a crucial set of regulations designed to ensure that all research data is publicly accessible. This initiative fosters openness and accountability in medical research, which is essential for maintaining public trust. These guidelines require sponsors to disclose comprehensive details about research studies, including study protocols, outcomes, and any adverse events. By doing so, the aim is to enhance public confidence in medical research and guarantee that all stakeholders - patients and healthcare professionals alike - have access to vital information regarding the safety and effectiveness of medical interventions.
These regulations align with broader European Union directives, particularly the EU Clinical Studies Regulation (EU No 536/2014). This regulation underscores the importance of transparency in research across member states, reinforcing the commitment to ethical standards in clinical trials. As the landscape of medical research evolves, these rules play a pivotal role in addressing key challenges and ensuring that the integrity of clinical studies is upheld.
In summary, the national clinical trials data transparency rules in Bulgaria are not just regulatory measures; they are a vital step towards fostering collaboration and trust in the medical research community. By prioritizing transparency, Bulgaria is setting a precedent that encourages responsible research practices and enhances the overall quality of clinical studies.
Transparency in clinical studies is not just beneficial; it is essential for building trust among researchers, participants, and the public. By openly sharing trial data, we enable independent verification of results, a cornerstone of scientific integrity. This level of openness significantly reduces the risks associated with selective reporting and publication bias, ensuring that all results - positive or negative - are available for scrutiny. Such transparency is particularly crucial for patient safety, empowering healthcare providers to make informed decisions based on comprehensive data.
Moreover, transparency enhances the effectiveness of medical research by minimizing redundancy and fostering collaboration among researchers. For instance, research indicates that 58% of products were fully compliant with FDAAA requirements, reflecting a growing commitment to openness in the field. This commitment not only advances healthcare knowledge but also significantly improves patient outcomes, as evidenced by the increased public trust in research practices.
In conclusion, embracing a transparent approach to medical studies is vital. It not only enriches the research landscape but also strengthens the foundation of trust necessary for effective healthcare delivery. As we move forward, collaboration among all stakeholders will be key to overcoming challenges and enhancing the integrity of clinical research.

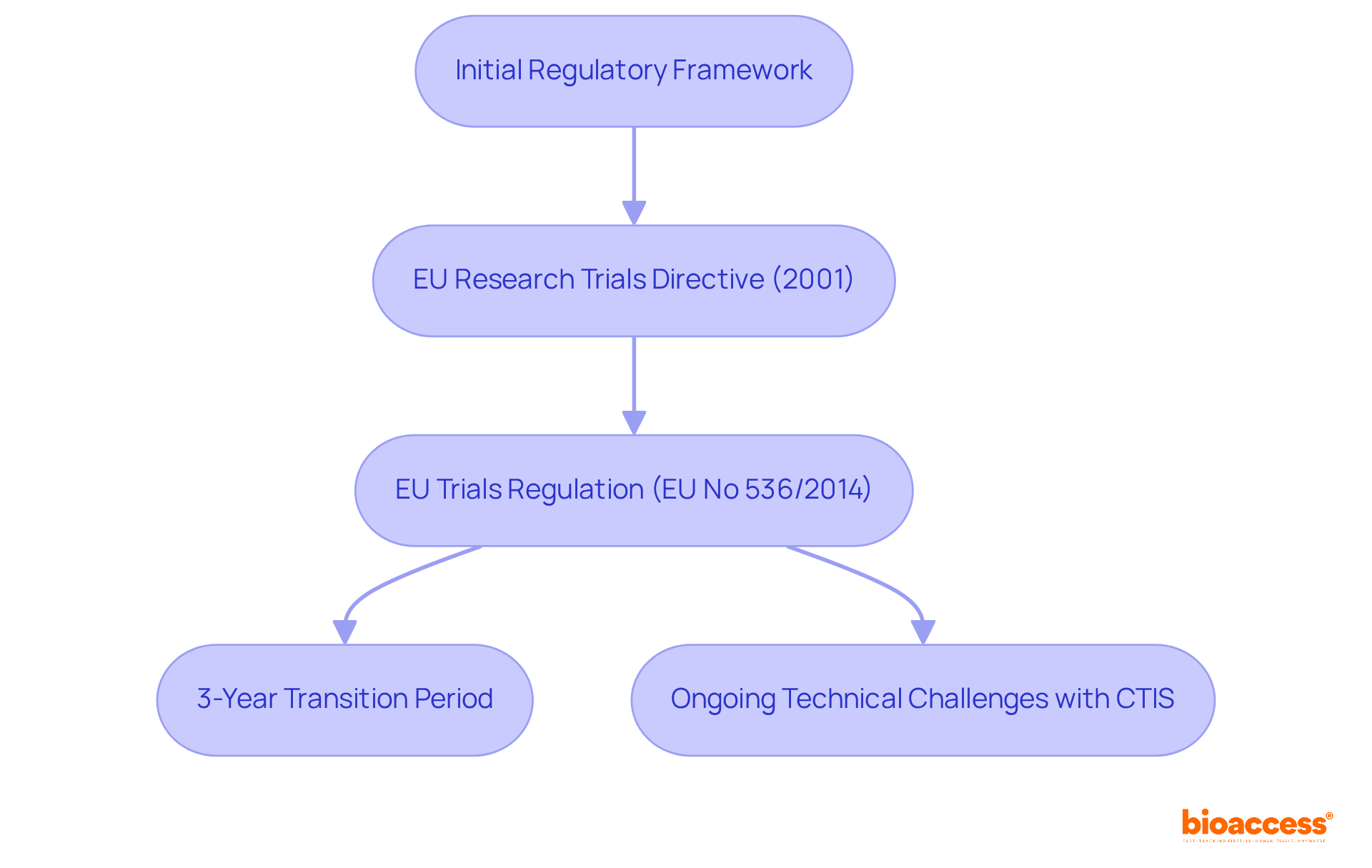
The national clinical trials data transparency rules in Bulgaria are a critical aspect of the clinical research landscape, significantly shaped by European Union policies. Initially, the regulatory framework featured minimal data disclosure requirements, which posed challenges for transparency. However, the introduction of the EU Research Trials Directive in 2001 marked a pivotal moment, laying the groundwork for enhanced openness in clinical trials. This directive was succeeded by the EU Trials Regulation (EU No 536/2014), which came into effect in January 2022. This regulation imposes stringent openness standards on all studies conducted within EU member states, including Bulgaria, in line with the national clinical trials data transparency rules in Bulgaria, mandating the publication of study protocols, results, and adverse events.
Notably, a three-year transition period allows companies to adapt to the new regulation, making necessary modifications and assessments. Despite these advancements, the Trial Information System (CTIS) has faced technical challenges since its launch, highlighting the ongoing need for improvement. This commitment to openness is vital, as it plays an essential role in safeguarding public health and fostering scientific progress. Furthermore, it aims to enhance the effectiveness of research studies across various member states, underscoring the importance of collaboration in navigating these complexities.
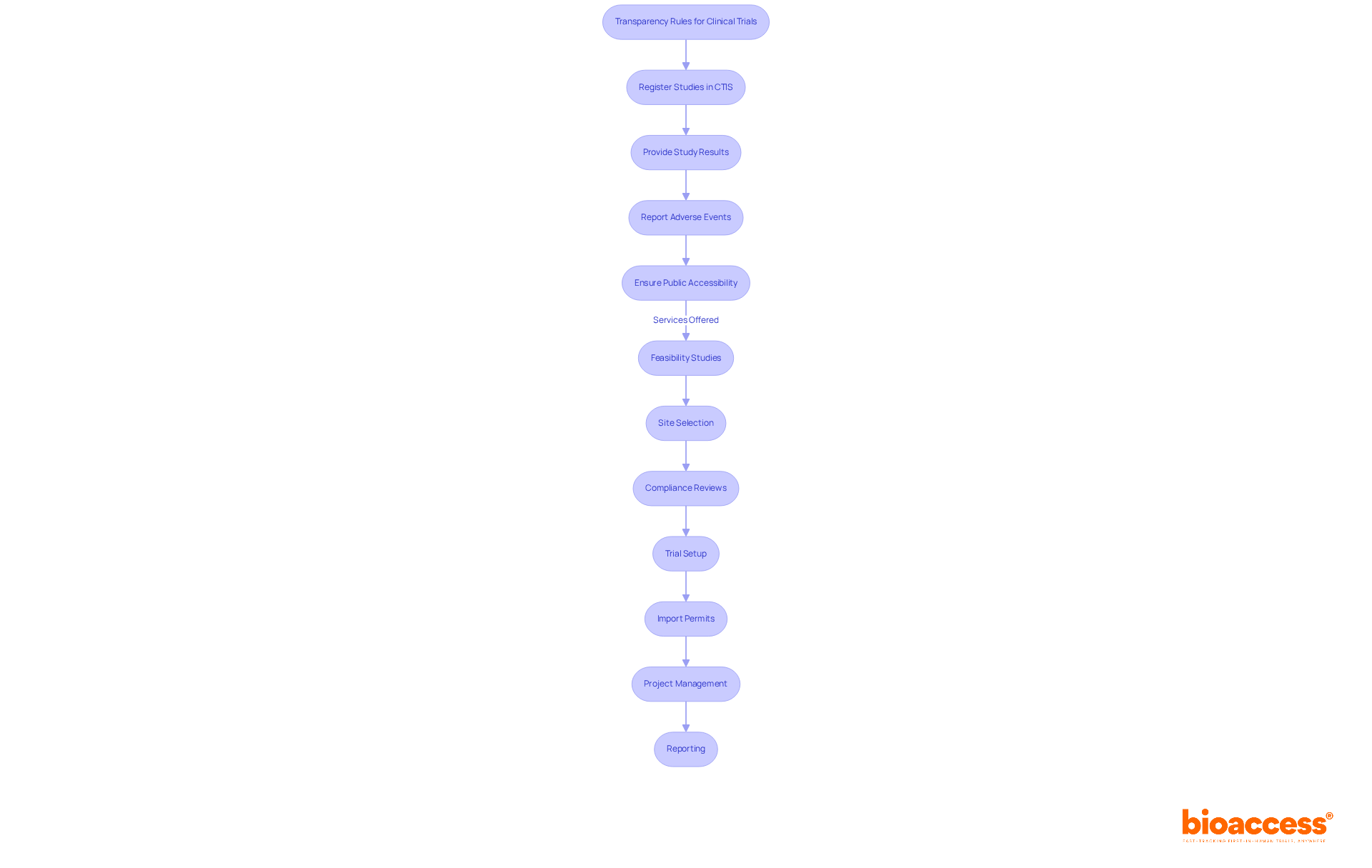
The national clinical trials data transparency rules in Bulgaria impose essential obligations on study sponsors, highlighting their critical role in clinical research. Firstly, sponsors must register their studies in the Clinical Trials Information System (CTIS) prior to recruitment. This registration is not merely a formality; it requires detailed information about the study's objectives, methodology, and anticipated outcomes, ensuring transparency from the outset.
Secondly, the regulations mandate that sponsors provide study results within a specified timeframe after completion. This includes a comprehensive outline of both efficacy and safety data, which is vital for informed decision-making. Furthermore, any adverse events must be reported transparently, effectively communicating potential risks to stakeholders. Such measures are designed to foster trust and accountability in the research process.
Additionally, the national clinical trials data transparency rules in Bulgaria stipulate that all information must be publicly accessible, facilitating thorough review and analysis by interested parties. Regulatory bodies actively oversee compliance with these requirements, reinforcing a commitment to high standards of openness in research activities.
At bioaccess, we recognize the significance of these regulations and offer comprehensive clinical trial management services tailored to meet these transparency requirements. Our services encompass:
By partnering with us, you enhance the integrity of your clinical research, ensuring adherence to regulatory standards while advancing your study's objectives.
The national clinical trials data transparency rules in Bulgaria mark a pivotal advancement in medical research, emphasizing openness and accountability. These regulations ensure that comprehensive information about clinical studies is accessible to all stakeholders, fostering trust and collaboration within the healthcare community. By mandating the disclosure of study protocols, outcomes, and adverse events, Bulgaria is taking decisive steps to bolster public confidence in the safety and efficacy of medical interventions.
Key aspects of these transparency rules have been explored, including their alignment with EU directives and their evolution from minimal disclosure requirements to stringent standards. The emphasis on timely registration and reporting by study sponsors is crucial; it not only promotes transparency but also safeguards patient safety. By reducing selective reporting and publication bias, these regulations encourage independent verification of results, ultimately benefiting both researchers and the public.
Reflecting on the importance of clinical trials data transparency, it is evident that these regulations are not merely bureaucratic measures; they are essential for advancing the integrity of medical research. As stakeholders in the healthcare ecosystem collaborate to navigate the complexities of clinical trials, a commitment to transparency will be vital. Embracing these principles can lead to improved patient outcomes and a more trustworthy research environment, making it imperative for all involved to prioritize and uphold these standards in their practices.
What are the national clinical trials data transparency rules in Bulgaria?
The national clinical trials data transparency rules in Bulgaria are regulations designed to ensure that all research data is publicly accessible, promoting openness and accountability in medical research.
Why are these transparency rules important?
These rules are important for maintaining public trust in medical research by ensuring that stakeholders, including patients and healthcare professionals, have access to vital information about the safety and effectiveness of medical interventions.
What information must sponsors disclose under these regulations?
Sponsors are required to disclose comprehensive details about research studies, including study protocols, outcomes, and any adverse events.
How do these rules align with European Union directives?
The regulations align with the EU Clinical Studies Regulation (EU No 536/2014), which emphasizes the importance of transparency in research across member states and reinforces ethical standards in clinical trials.
What role do these rules play in the medical research landscape?
These rules are crucial for addressing key challenges in medical research and ensuring the integrity of clinical studies, while fostering collaboration and trust within the medical research community.
What is the overall goal of the national clinical trials data transparency rules in Bulgaria?
The overall goal is to enhance public confidence in medical research and encourage responsible research practices, ultimately improving the quality of clinical studies.