


Orphan diseases are classified as rare medical conditions that impact fewer than 200,000 individuals in the U.S. These conditions are marked by their low prevalence and pose significant challenges in terms of diagnosis and treatment. The implications of these diseases on healthcare systems are profound, underscoring the necessity for robust research and development efforts.
Regulatory frameworks, such as the Orphan Drug Act, have catalyzed innovation and investment in effective therapies, addressing the needs of these conditions that collectively affect millions worldwide.
Orphan diseases, defined as rare medical conditions affecting fewer than 200,000 individuals in the U.S., present unique challenges that often result in significant gaps in research and treatment options. With millions globally impacted by these conditions, the urgency for effective therapies has never been greater.
What drives the development of innovative solutions in this neglected area of healthcare? Moreover, how do regulatory frameworks shape the landscape for research and patient care?
These questions underscore the critical need for focused attention and action in addressing the complexities of orphan diseases.
According to the Orphan Drug Act of 1983, an orphan disease is defined as a rare medical issue affecting fewer than 200,000 individuals in the United States. Worldwide, although definitions may differ, orphan diseases are characterized by their low prevalence, which frequently leads to restricted research and treatment alternatives. Serious in nature and often life-threatening, orphan diseases present significant challenges in diagnosis and management.
For instance, cystic fibrosis and Huntington's condition, despite their rarity, necessitate specialized medical attention and resources. Recent studies indicate that approximately 30 million individuals in the U.S. live with an orphan disease, highlighting the urgent need for effective treatments and research initiatives. The occurrence of uncommon health conditions globally is estimated to impact between 260 million and 440 million individuals, highlighting the extensive effect of these ailments.
In the European Union, conditions with fewer than 50 cases per 100,000 individuals are classified as orphan diseases, with nearly 7,000 such conditions currently estimated. As the field of neglected illness research progresses, effective therapies for orphan diseases are becoming available, with over 500 designated medications commercially accessible worldwide for various uncommon conditions.
Bioaccess® plays a crucial role in this evolving landscape by providing accelerated clinical trial services, including regulatory approval, clinical research site activation, and patient recruitment. This capability enables innovative Medtech, Biopharma, and Radiopharma startups to navigate the complexities of clinical trials efficiently, moving to the next phase of studies up to 40% faster, ultimately enhancing the potential for advancements in this critical area of healthcare.
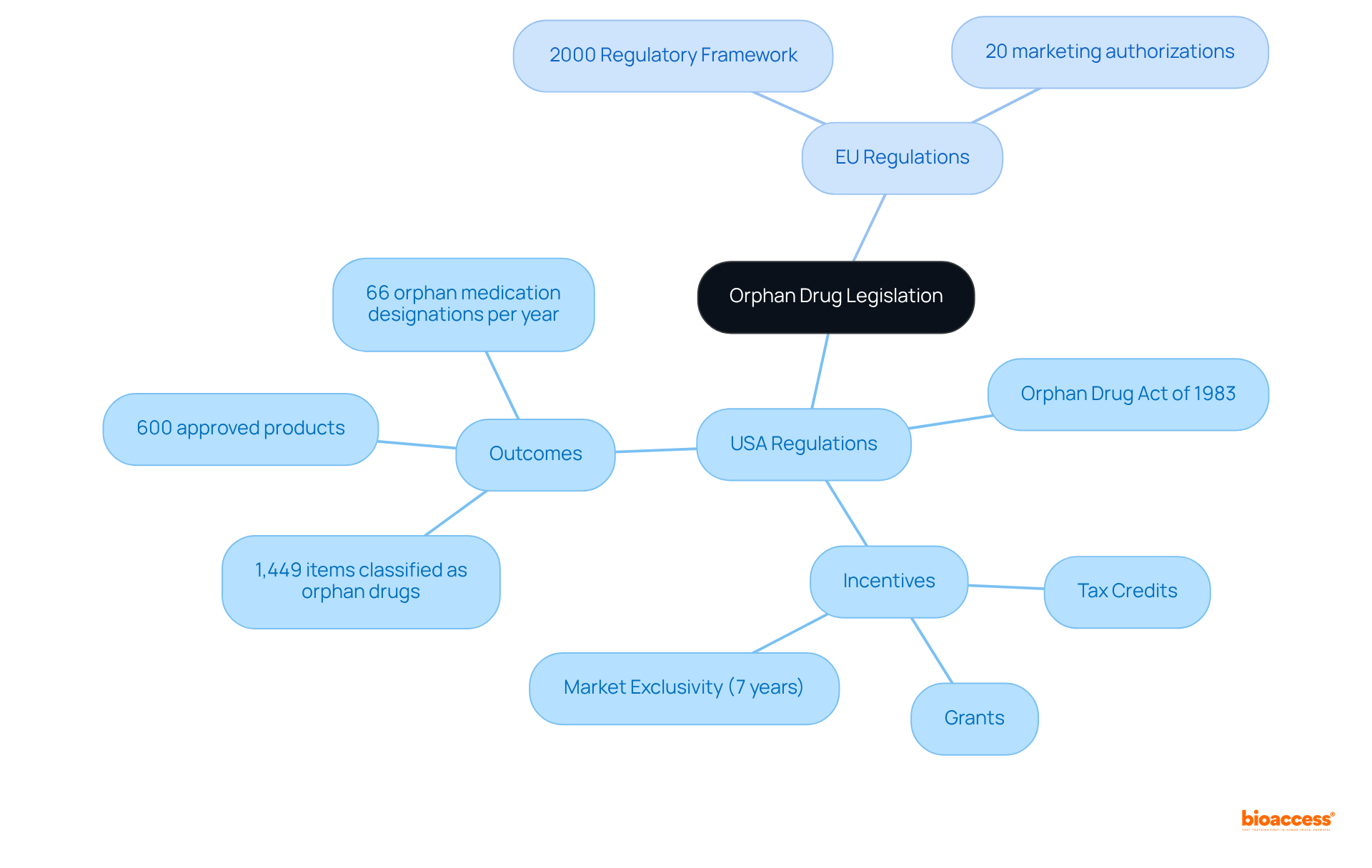
The historical context of previously neglected conditions transformed with the introduction of the Orphan Drug Act in the United States in 1983, which focused on orphan disease. This landmark legislation aimed to stimulate the development of therapies for orphan diseases by offering pharmaceutical firms a variety of incentives, such as tax credits, grants, and a seven-year market exclusivity period for specialized medications.
Since the enactment of the Orphan Product Act, 1,449 items have been classified as rare treatments in the USA, with 269 receiving FDA endorsement as of April 2005. Consequently, the Act has led to the approval of over 600 products and biologic items for orphan disease, significantly enhancing research and development in this critical field.
In parallel, the European Union established its regulatory framework for rare medicinal products in 2000, mirroring the U.S. approach. Notably, 20 rare therapeutic agents (7%) out of 287 designations have secured marketing authorizations in the EU, underscoring the effectiveness of these regulations.
These regulatory measures have been pivotal in fostering innovation and attracting investment in treatments for orphan diseases, which were historically overlooked due to their limited commercial viability. The average number of orphan medication designations per year in the USA stands at 66, and the percentage of sponsors intending to submit applications for marketing approval increased from 9% in 2005 to 17% in 2008. This trend illustrates a growing recognition of the necessity for effective therapies for uncommon conditions affecting millions worldwide.
As Laura Karas observes, 'The Orphan Drug Act of 1983 (ODA) established a series of financial and marketing incentives to encourage the development of medications for orphan diseases.' Furthermore, an illness classified as an orphan disease in the USA is one that impacts fewer than 200,000 people, which corresponds to approximately 7.5 per 10,000 of the population.
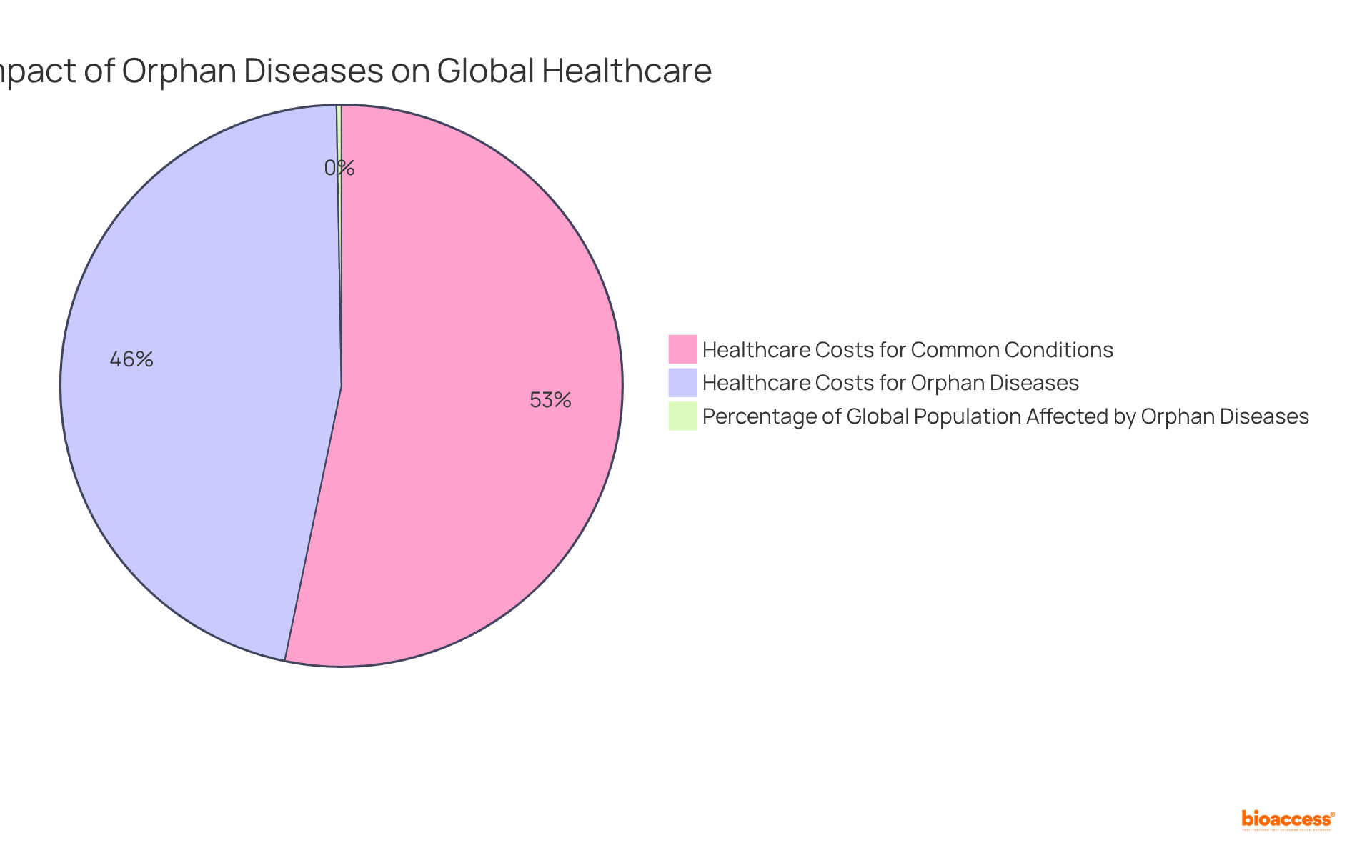
Orphan diseases collectively impact a substantial segment of the global population, with estimates indicating that between 3.5% and 5.9% of individuals worldwide are affected by these rare health issues. This prevalence translates to over 30 million individuals in Europe alone, who confront unique healthcare challenges associated with orphan diseases, including:
The implications for healthcare systems are profound, as these conditions often require multidisciplinary approaches and long-term management strategies. For instance, the average duration of hospital stays for patients with uncommon conditions is 6.3 days, compared to 3.8 days for those with typical ailments, underscoring the complexity of care needed.
Financially, the burden associated with neglected illnesses is staggering, with total aggregate healthcare expenses reaching approximately $768 billion in 2016—almost on par with the costs for prevalent conditions, which amounted to $880 billion. This financial strain can escalate costs for both patients and providers, highlighting the necessity for effective resource allocation.
Policymakers and healthcare professionals must grasp this prevalence and its implications to improve patient care and optimize healthcare resource distribution.
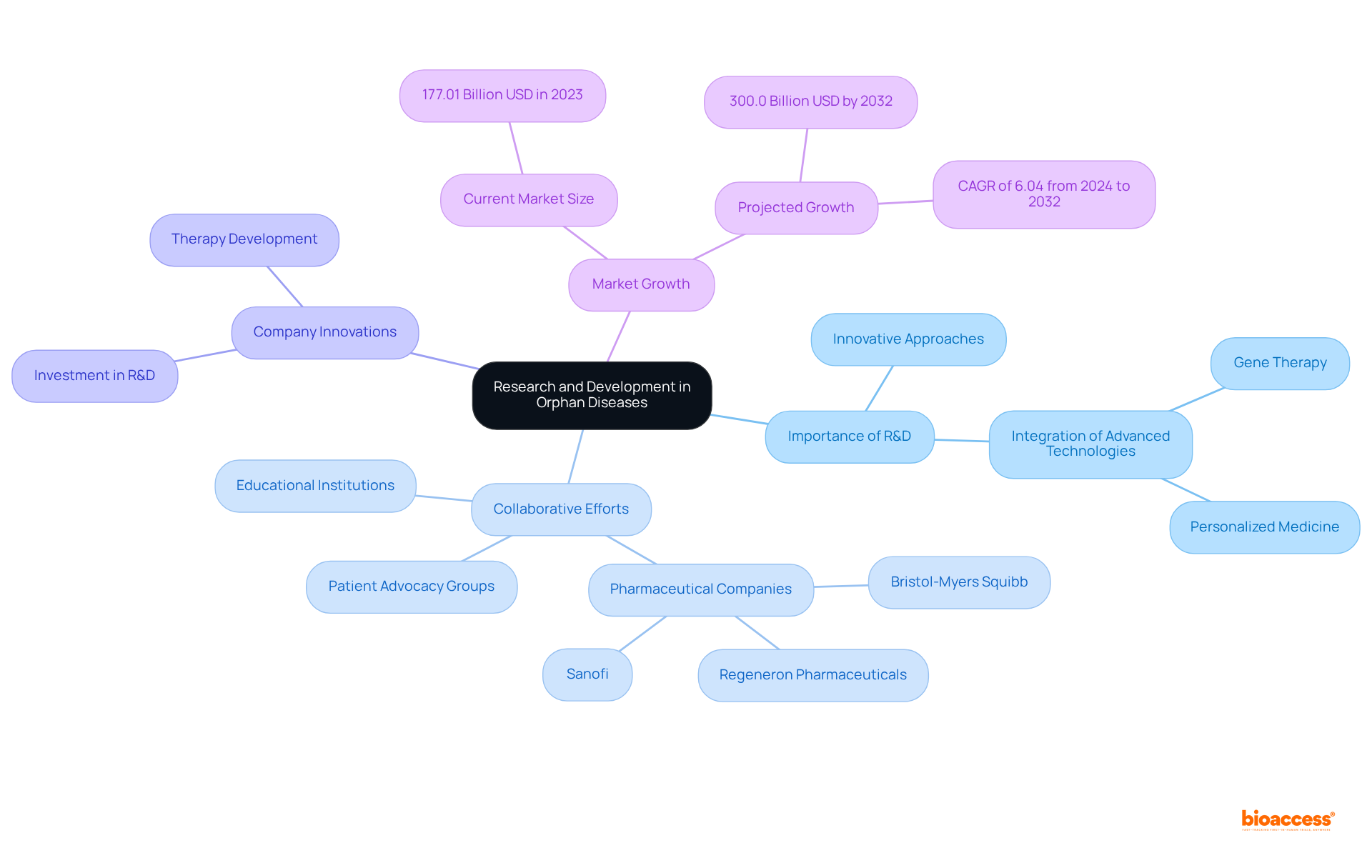
Research and development are pivotal in addressing the challenges posed by orphan diseases. The unique characteristics of these conditions often necessitate innovative approaches in pharmaceutical development, integrating advanced technologies such as gene therapy and personalized medicine. Collaborative efforts among educational institutions, pharmaceutical companies, and patient advocacy groups have led to significant progress in understanding the underlying mechanisms of these conditions.
For example, Luke Rosen, a patient advocate, underscores the necessity of creating lexicons that render scientific advancements accessible to the public. Furthermore, the establishment of rare disease drug designations has catalyzed investment in R&D, resulting in a growing pipeline of therapies aimed at improving patient outcomes.
Companies like Bristol-Myers Squibb and Sanofi are actively pursuing innovative strategies for treating rare conditions, showcasing their commitment to addressing the needs of patients with uncommon illnesses. Notably, the Orphan Diseases Market is projected to expand from 177.01 billion USD in 2023 to 300.0 billion USD by 2032, reflecting a compound annual growth rate (CAGR) of 6.04% from 2024 to 2032.
Ongoing support for research initiatives is crucial to ensure that individuals affected by orphan diseases receive the necessary care and treatment, ultimately improving their quality of life.
Orphan diseases pose a significant challenge within the healthcare landscape, affecting millions of individuals globally, yet often lacking sufficient research and treatment options. The urgency for effective therapies and innovative solutions in this neglected area is paramount, as these rare conditions impact not only the patients but also the healthcare systems striving to support them.
Throughout this discussion, critical insights have emerged regarding the definition and prevalence of orphan diseases, the historical context of regulatory frameworks, and the essential role of research and development. The Orphan Drug Act has spurred advancements in treatment options, while ongoing research efforts continue to deepen understanding and management of these conditions. Notably, the financial burden associated with orphan diseases underscores the necessity for effective resource allocation and multidisciplinary care approaches.
Addressing orphan diseases transcends medical necessity; it is a moral imperative. Stakeholders—including policymakers, healthcare professionals, and researchers—must collaborate to ensure that individuals affected by these conditions receive the attention and care they rightfully deserve. By fostering innovation and investing in research, we can unlock the potential for breakthroughs in treatment, ultimately enhancing the quality of life for those impacted by orphan diseases.
What is an orphan disease?
An orphan disease is defined as a rare medical issue affecting fewer than 200,000 individuals in the United States, as per the Orphan Drug Act of 1983. These diseases are characterized by their low prevalence, leading to limited research and treatment options.
How many people in the U.S. are affected by orphan diseases?
Approximately 30 million individuals in the U.S. live with an orphan disease.
What are some examples of orphan diseases?
Examples of orphan diseases include cystic fibrosis and Huntington's disease, both of which require specialized medical attention and resources despite their rarity.
How do orphan diseases impact individuals globally?
Globally, orphan diseases are estimated to affect between 260 million and 440 million individuals, indicating their widespread impact.
What is the classification of orphan diseases in the European Union?
In the European Union, conditions with fewer than 50 cases per 100,000 individuals are classified as orphan diseases, with nearly 7,000 such conditions currently estimated.
What advancements have been made in the treatment of orphan diseases?
Over 500 designated medications for various uncommon conditions are commercially available worldwide, indicating progress in the development of effective therapies for orphan diseases.
How does Bioaccess® contribute to the research and treatment of orphan diseases?
Bioaccess® provides accelerated clinical trial services, including regulatory approval, clinical research site activation, and patient recruitment, helping Medtech, Biopharma, and Radiopharma startups navigate clinical trials more efficiently, which can enhance advancements in healthcare for orphan diseases.