


This article elucidates the pivotal role and responsibilities of a Principal Investigator (PI) in research, emphasizing their essential functions in project management, ethical compliance, and team leadership.
It details the myriad responsibilities PIs undertake, including:
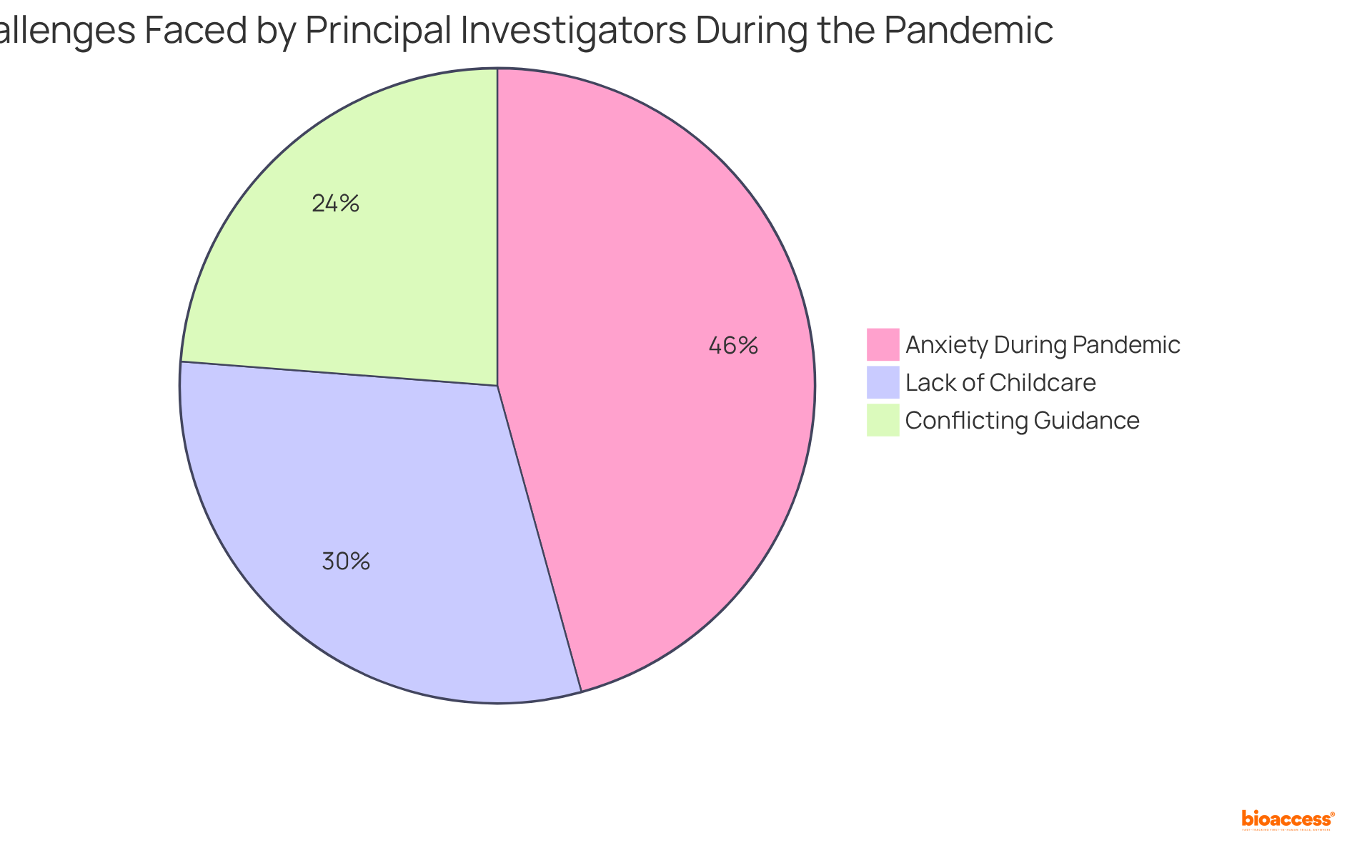
Additionally, it addresses the challenges they encounter, such as:
These challenges are crucial for the success of clinical research initiatives. The significance of these roles cannot be overstated, as they are integral to navigating the complexities of clinical research and ensuring its integrity and efficacy.
The role of a Principal Investigator (PI) is central to the success of research projects, yet it often remains shrouded in complexity and misunderstanding. As the driving force behind study design, regulatory compliance, and team leadership, PIs navigate a landscape fraught with challenges, from administrative burdens to evolving technological demands. This article delves into the multifaceted responsibilities of PIs, exploring not only their critical functions but also the skills and qualifications necessary to excel in this pivotal role.
How can PIs effectively balance these demands while advancing scientific inquiry in an increasingly competitive environment?
A Principal Investigator (PI) meaning research serves as the pivotal individual responsible for the comprehensive design, execution, and management of a research project. Typically a faculty member or senior investigator with an independent grant, the principal investigator leads the team and plays an essential role in ensuring that the study adheres to ethical standards and regulatory requirements. This position is crucial for steering the project toward successful outcomes, as the PI is often viewed as the face of the initiative, representing it to funding organizations, regulatory bodies, and the scientific community.
Recent statistics reveal that over 40% of active PIs in the U.S. conducted only one industry-sponsored pharmaceutical clinical trial from 2018 to 2020, highlighting a significant trend where many investigators engage minimally with industry-sponsored activities. Furthermore, approximately 20.3% of U.S. investigators fall into the 'one and done' category, indicating participation in a single trial. This trend underscores the challenges faced by PIs, including heightened administrative burdens and cash flow issues, which may discourage them from pursuing additional studies. Notably, 80% of nearly 37,600 active PIs managed just one to five trials with industry funding during the same three-year span, illustrating the limited involvement of many PIs in industry-sponsored research.
In the Medtech sector, PIs are vital for fostering innovation and ensuring the successful execution of research trials. Their responsibilities include designing study protocols, overseeing data collection, and safeguarding participant safety, all while upholding rigorous scientific and regulatory standards. As the landscape of medical research evolves, the role of PIs becomes increasingly critical in navigating the complexities of modern trials, particularly in the context of pi meaning research related to emerging technologies and methodologies. The number of U.S. PIs conducting pharmaceutical trials was 28,292 in 2014 and 26,115 in 2020, indicating a stable yet demanding environment for research investigators. Additionally, as highlighted by Harold E. Glass, various factors such as increased administrative burdens and cash flow challenges may lead PIs to refrain from conducting further studies.
bioaccess® significantly contributes to this landscape by providing ethical approvals in 4-6 weeks and achieving enrollment 50% faster than traditional markets, thereby supporting PIs in their essential roles.
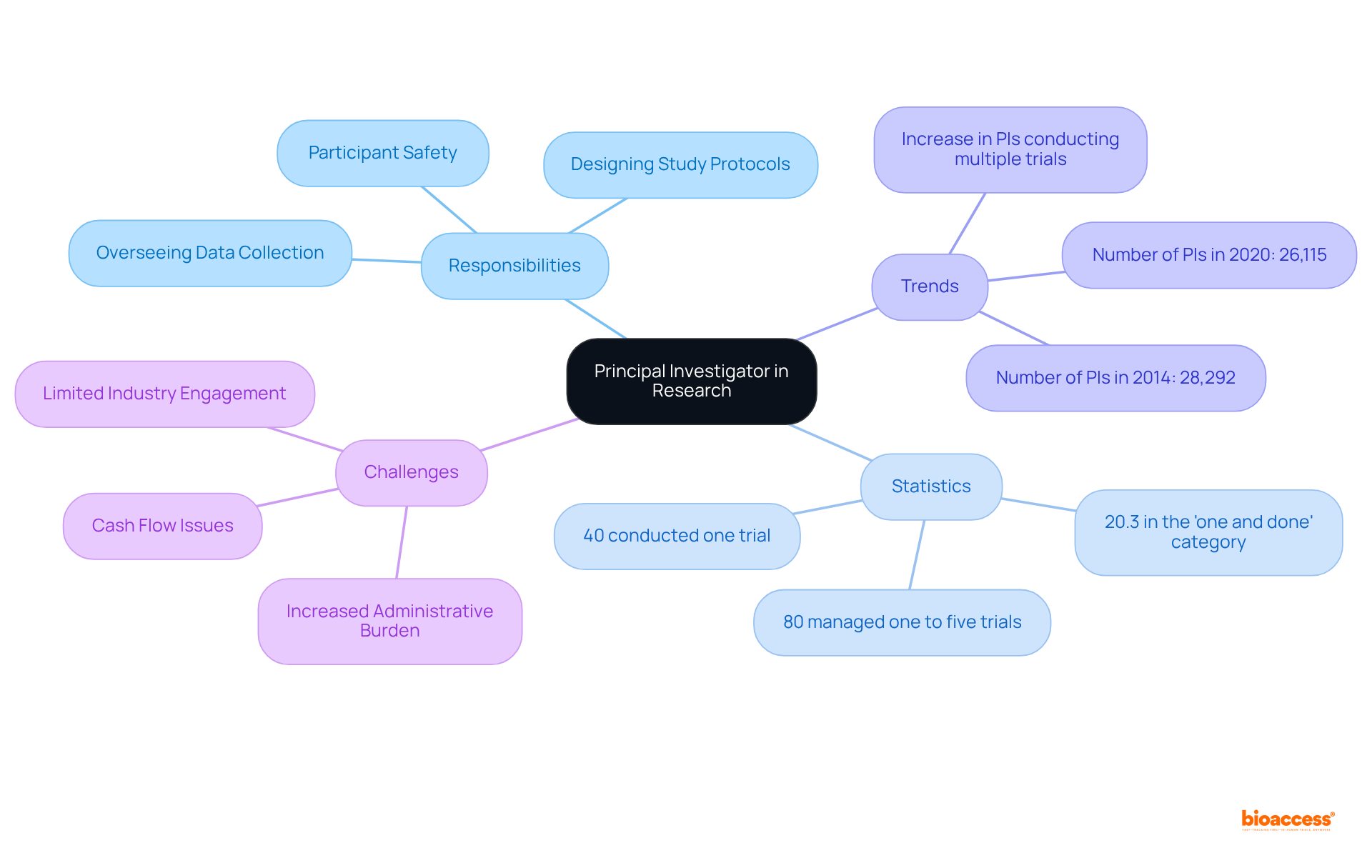
The role of a Principal Investigator (PI) is embedded in a multifaceted landscape of clinical inquiry, necessitating collaboration with various stakeholders, including sponsors, regulatory bodies, and ethics committees. PIs typically operate within academic institutions, hospitals, or contract clinical organizations (CROs), where they must skillfully navigate institutional policies and federal regulations. Their guidance is vital in fostering a culture of integrity and adherence, ensuring that the rights and welfare of participants are prioritized.
Recent statistics reveal that:
As the funding landscape evolves, PIs must adapt to new trends and technological advancements that can significantly influence study design and execution. For instance, the incorporation of artificial intelligence in medical trials is anticipated to boost compliance monitoring and enhance overall trial efficiency by 2025.
Collaboration remains essential for PIs, as it promotes innovation and enables access to resources, ultimately aiding the successful progression of clinical projects. As pointed out by Kari Baldwin, PIs had to make choices regarding carrying out and staffing vital studies under unprecedented circumstances, highlighting the flexibility needed in the present environment.
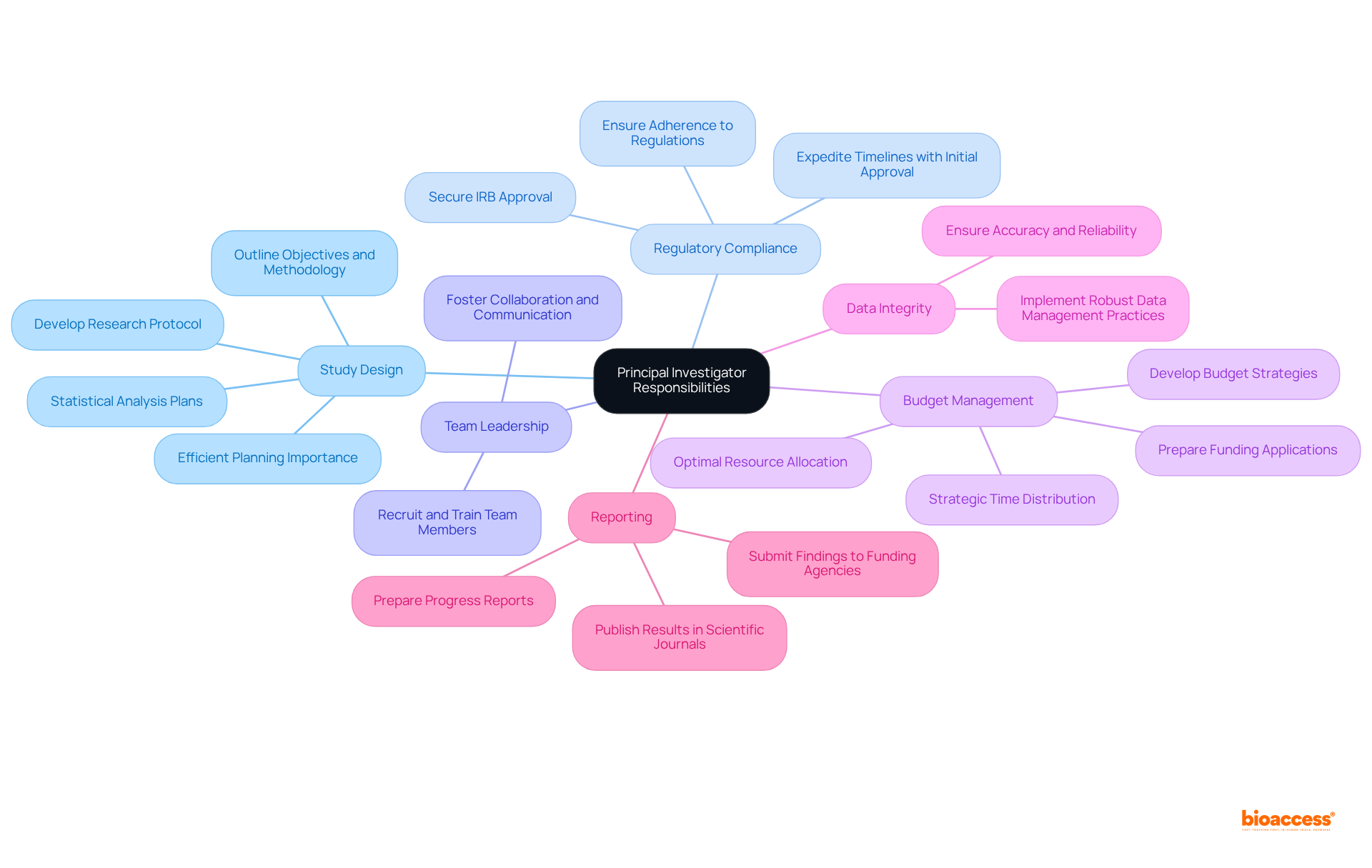
The Principal Investigator (PI) is integral to the success of research projects, encompassing several key responsibilities:
Study Design: The PI is tasked with developing a comprehensive research protocol that outlines the project's objectives, methodology, and statistical analysis plans. This foundational step is crucial for ensuring scientific rigor. Notably, the average time spent by PIs on study design can be substantial, underscoring the significance of efficient planning.
Regulatory Compliance: A vital aspect of the PI's role is ensuring adherence to all applicable regulations and ethical guidelines. This includes securing Institutional Review Board (IRB) approval, essential for safeguarding the rights and welfare of study participants. Studies that obtain IRB approval on the initial submission can significantly expedite timelines, enhancing overall efficiency.
Team Leadership: The PI is responsible for recruiting, training, and managing the study team. Effective leadership fosters collaboration and communication among team members, which is essential for maintaining a productive working environment.
Budget Management: Overseeing the financial aspects of the study is another critical responsibility. This includes developing budget management strategies, preparing funding applications, and ensuring optimal resource allocation to meet project goals. Given that additional time spent on proposals did not correlate with a higher likelihood of success, strategic time distribution in budget management is crucial for enhancing productivity in studies.
Data Integrity: Ensuring the accuracy and reliability of data collected during the study is paramount. The PI must uphold proper documentation and implement robust data management practices to maintain the integrity of the findings.
Reporting: The PI is also responsible for preparing and submitting progress reports to funding agencies and stakeholders. Additionally, sharing study results through publication in scientific journals is essential for contributing to the broader scientific community.
By efficiently overseeing these responsibilities, Principal Investigators not only advance their investigative goals but also enhance the overall integrity and success of the scientific enterprise. As highlighted by Danielle L Herbert, scholars prefer to allocate less time to preparing grant applications and more time to actual inquiries, emphasizing the necessity for effective procedures in project management.
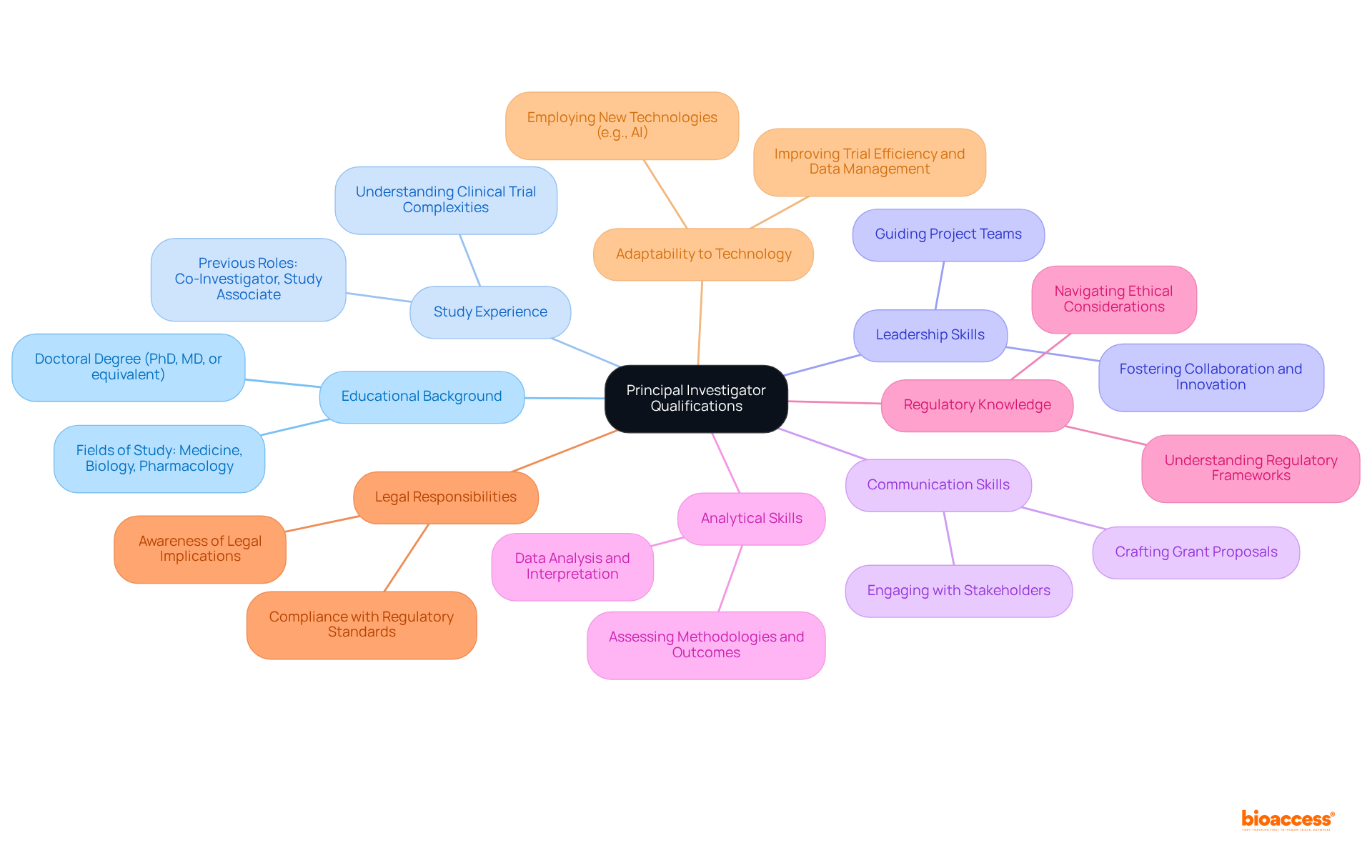
To excel as a Principal Investigator (PI), individuals must possess a blend of education, experience, and essential skills, including:
Educational Background: Most PIs possess a doctoral degree (PhD, MD, or equivalent) in areas such as medicine, biology, or pharmacology, which offers a strong basis for medical studies.
Study Experience: Successful PIs typically possess extensive study experience, often having served as co-investigators or study associates prior to assuming the PI role. This background is crucial for understanding the complexities of clinical trials.
Leadership Skills: Strong leadership and management abilities are essential for guiding project teams effectively and fostering a collaborative environment that encourages innovation and productivity.
Communication Skills: Excellent verbal and written communication skills are necessary for engaging with stakeholders, presenting findings, and crafting compelling grant proposals. These skills are crucial for ensuring that goals are clearly articulated and understood.
Analytical Skills: Expertise in data analysis and interpretation is essential, as PIs must assess methodologies and outcomes rigorously. This analytical ability allows them to make informed choices during the inquiry process.
Regulatory Knowledge: A thorough grasp of the regulatory framework overseeing medical studies is essential. PIs must navigate ethical considerations and compliance requirements to uphold the integrity of their studies and protect participant rights.
Legal Responsibilities: PIs must be aware of the legal implications of their role, including the potential consequences for failing to comply with regulatory standards or engaging in unethical practices.
Adaptability to Technology: As medical studies progress, PIs should be skilled at employing new technologies, such as AI-driven analytics, to improve trial efficiency and data management.
The combination of these qualifications not only enhances the effectiveness of PIs but also contributes to the overall success and ethical execution of clinical research initiatives. Notably, the Clinical Investigator Training Program (CITP) has shown a statistically significant improvement in mean post-test scores (p < 0.01), and the number of participants achieving first-time principal investigator status nearly doubled from 17 to 33, underscoring the importance of training in preparing PIs for their roles.
The role of a Principal Investigator (PI) in research is foundational to the success and integrity of clinical trials. PIs are not only responsible for the design and execution of research projects but also play a critical role in ensuring compliance with ethical standards and regulatory requirements. Their leadership is essential in navigating the complexities of modern medical research, making their contributions invaluable to the scientific community.
Key insights presented throughout this article highlight the responsibilities and challenges faced by PIs:
In conclusion, understanding the significance of the Principal Investigator's role underscores the necessity for proper support and resources to enable these professionals to thrive. As the landscape of research continues to evolve, fostering innovation and ensuring ethical practices will be paramount. Stakeholders in the research community must recognize the challenges faced by PIs and work collaboratively to create an environment that empowers them to lead successful and impactful studies. Emphasizing the importance of training and support systems will not only enhance the individual capabilities of PIs but also contribute to the advancement of medical research as a whole.
What is the role of a Principal Investigator (PI) in research?
A Principal Investigator (PI) is responsible for the comprehensive design, execution, and management of a research project. They lead the research team and ensure that the study adheres to ethical standards and regulatory requirements.
Who typically holds the position of a Principal Investigator?
The position of Principal Investigator is typically held by a faculty member or senior investigator who has an independent grant.
What are some challenges faced by Principal Investigators in the U.S.?
Challenges faced by PIs include heightened administrative burdens and cash flow issues, which may discourage them from pursuing additional studies. Many PIs engage minimally with industry-sponsored activities.
How many clinical trials do most Principal Investigators manage?
Approximately 80% of active PIs managed just one to five trials with industry funding from 2018 to 2020, indicating limited involvement in industry-sponsored research.
What is the significance of Principal Investigators in the Medtech sector?
In the Medtech sector, PIs are vital for fostering innovation and ensuring the successful execution of research trials. They design study protocols, oversee data collection, and ensure participant safety while maintaining scientific and regulatory standards.
How has the number of U.S. Principal Investigators conducting pharmaceutical trials changed over time?
The number of U.S. PIs conducting pharmaceutical trials was 28,292 in 2014 and decreased to 26,115 in 2020, indicating a stable yet demanding environment for research investigators.
What support does bioaccess® provide to Principal Investigators?
bioaccess® contributes by providing ethical approvals in 4-6 weeks and achieving enrollment 50% faster than traditional markets, thereby supporting PIs in their essential roles.