


Understanding the regulatory landscape of gene therapy trials is crucial as this innovative medical field continues to evolve. In Serbia, the framework governing these trials emphasizes not only safety and efficacy but also aligns with EU standards, fostering an environment ripe for research and development. As the country gears up for 2025, a significant challenge emerges: how can sponsors effectively navigate the complexities of regulatory compliance while maximizing the benefits of conducting trials in this emerging hub for gene therapy? This question invites stakeholders to consider their strategies and approaches in this dynamic landscape.
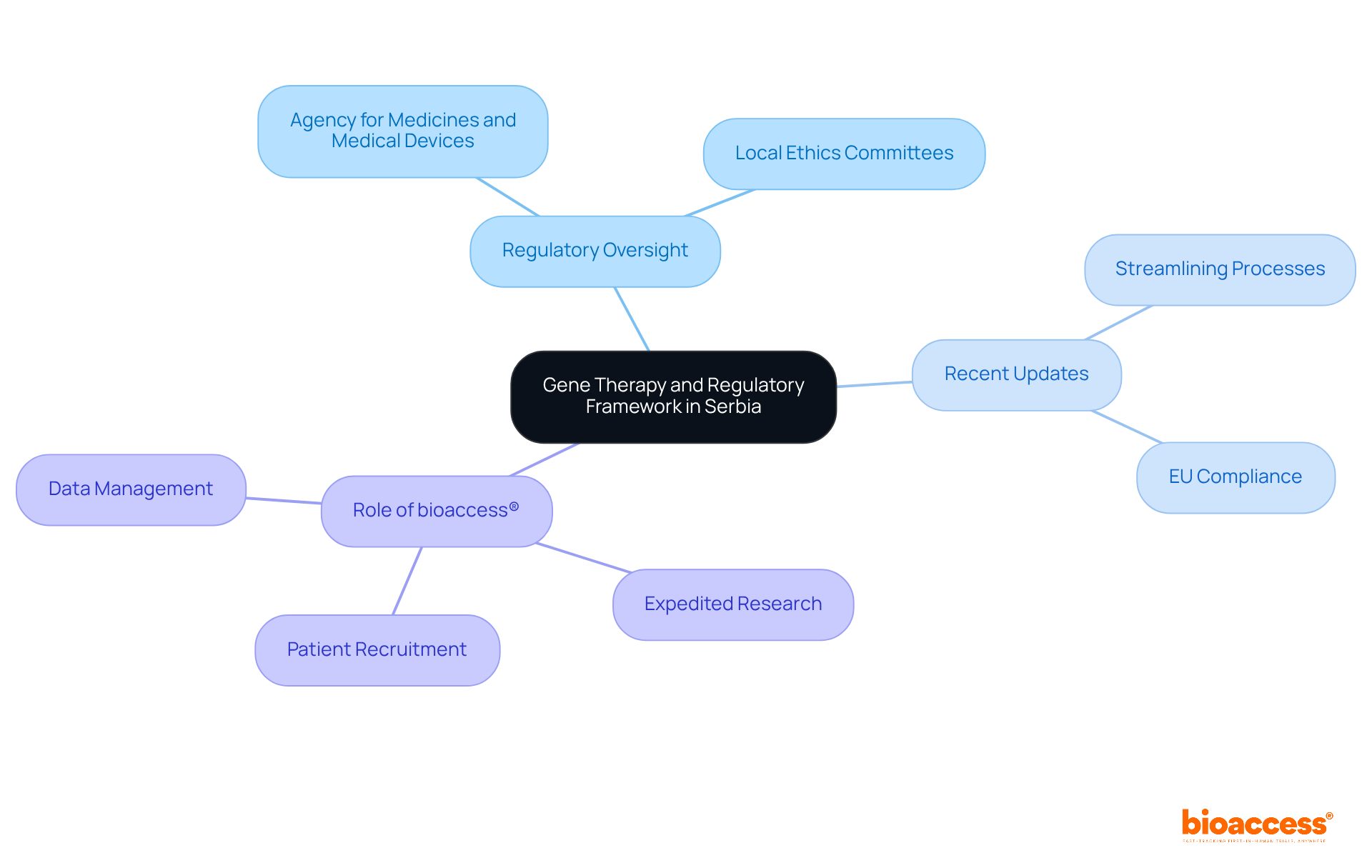
Gene treatment represents a significant advancement in medical science, involving the alteration of genetic material to address or prevent diseases. In this context, the regulatory oversight of gene therapy trials in Serbia is primarily based on the Medicines and Medical Devices Act, which is designed to align with EU regulations. This framework ensures that therapy experiments are conducted ethically and safely, with the regulatory oversight of gene therapy trials in Serbia provided by the Agency for Medicines and Medical Devices and local ethics committees.
Recent updates to the Medicines Act reflect the country’s commitment to fostering innovation in genetic therapy by streamlining regulatory processes. These amendments enhance compliance with EU standards, paving a more efficient path for conducting clinical studies. As we look toward 2025, the nation is solidifying its position as a favorable environment for genetic therapy research, with successful trials demonstrating its capacity to support advanced therapeutic developments.
The regulatory oversight of gene therapy trials in Serbia emphasizes safety and efficacy, ensuring that all therapy products undergo rigorous evaluation before market entry. This dedication to high standards is essential for maintaining public trust and advancing the regulatory oversight of gene therapy trials in Serbia.
In this framework, bioaccess® plays a pivotal role by delivering expedited research study results, ensuring swift and reliable regulatory approvals, research site activation, patient recruitment, and study data management. By connecting innovative Medtech, Biopharma, and Radiopharma startups with leading clinical research sites in the region, bioaccess® enhances the effectiveness of progressing to the next stage of clinical studies, securing funding, and achieving successful exits.
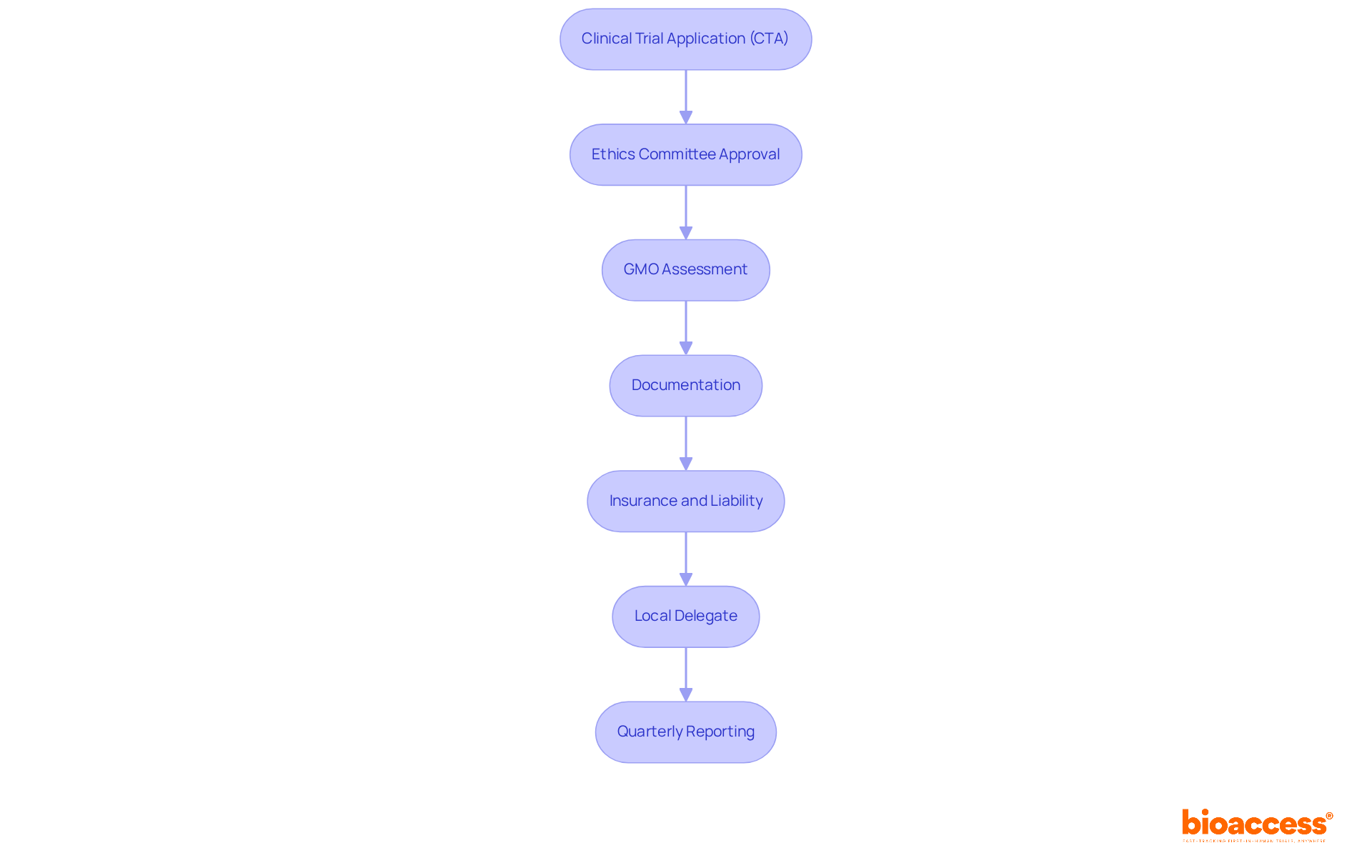
Conducting gene therapy trials in Serbia requires strict adherence to several essential regulatory requirements:
Clinical Trial Application (CTA): Sponsors must submit a comprehensive application to the Agency for Medicines and Medical Devices of Serbia (ALIMS), which includes detailed study protocols and informed consent forms. Recent data shows that approximately 90 studies for medications and medical devices gain approval each year in Serbia, reflecting a robust clinical research environment. Bioaccess offers invaluable support in the feasibility and selection of research sites and principal investigators (PIs), streamlining this process.
Ethics Committee Approval: Securing approval from a local ethics committee is mandatory. This step ensures that the experiment adheres to ethical standards, with most applications generally authorized within 60 days. Bioaccess provides thorough review and feedback on study documents to meet local requirements, fostering a supportive environment for research.
GMO Assessment: If the genetic treatment involves genetically altered organisms, a separate GMO application is required. This application must be submitted alongside the CTA, as genetic therapy studies often face additional regulatory layers and extended timelines. Bioaccess assists in navigating these complexities to ensure compliance.
Documentation: All essential documents must be provided in both Serbian and English, including patient-related materials and study protocols. This bilingual requirement facilitates clear communication and compliance with local regulations, a service that Bioaccess can help manage.
Insurance and Liability: Sponsors are responsible for ensuring adequate insurance coverage for participants in the study, addressing potential risks associated with gene therapy. This is crucial for safeguarding participant welfare and maintaining ethical standards throughout the research process. Bioaccess emphasizes the importance of this aspect in its project management services.
Local Delegate: Designating a local delegate is crucial, as ALIMS will not accept the research application without one. This representative plays a critical role in navigating the regulatory landscape, and Bioaccess can assist in identifying suitable candidates.
Quarterly Reporting: Sponsors are required to provide quarterly updates on study progress to ALIMS, ensuring continuous compliance and oversight. Bioaccess offers comprehensive reporting services, including study status updates and inventory management, to support sponsors in meeting these obligations.
By following these requirements and utilizing the comprehensive clinical study management services offered by Bioaccess, sponsors can navigate the regulatory oversight of gene therapy trials in Serbia efficiently, ensuring successful study initiation and compliance with Serbian regulations.
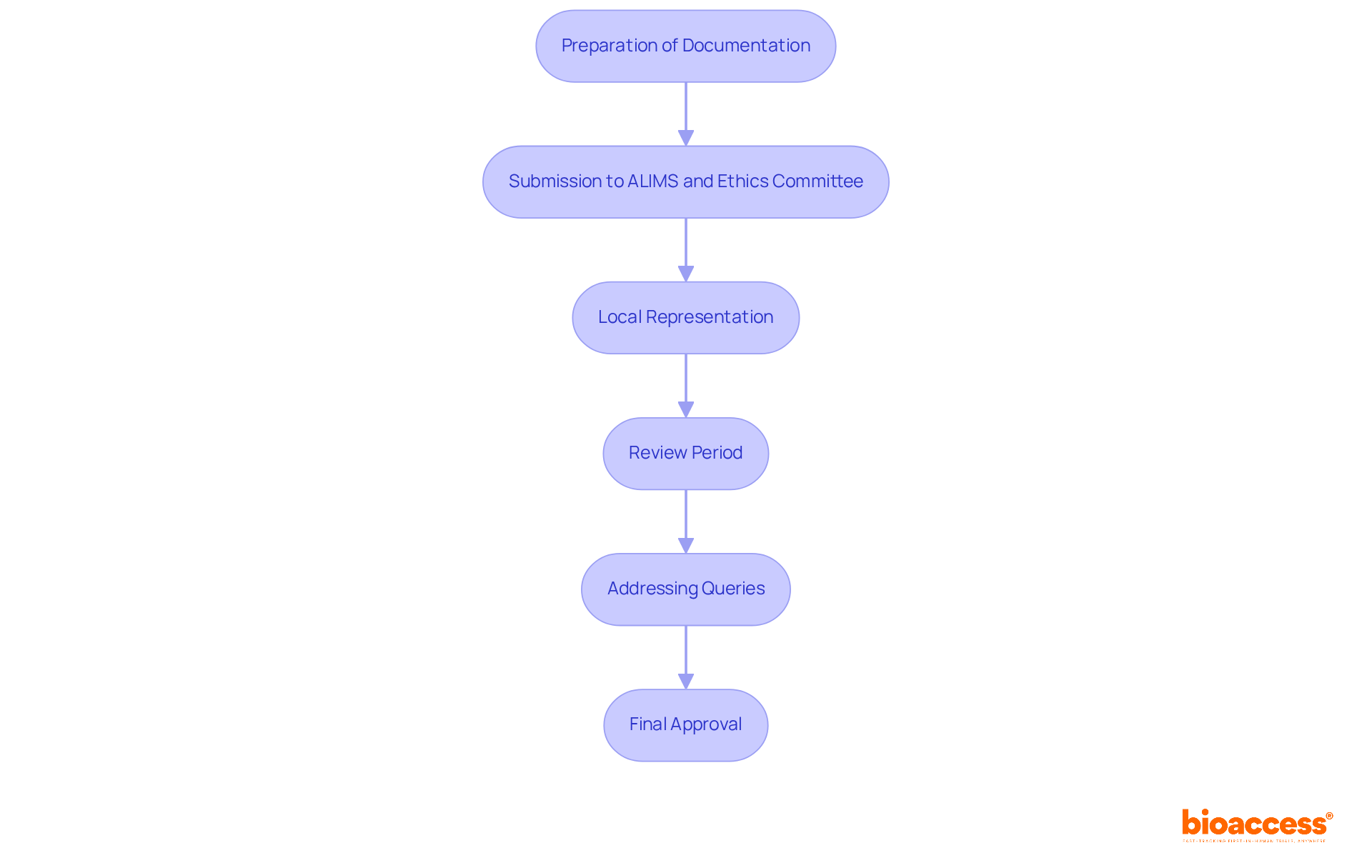
Navigating the regulatory oversight of gene therapy trials in Serbia is crucial for advancing clinical research. This process involves several key steps that ensure compliance and efficiency.
Preparation of Documentation: Start by compiling all necessary documents, including the Clinical Trial Application (CTA), ethics approval, and any required Genetically Modified Organism (GMO) assessments. This foundational step sets the stage for a successful application.
Submission to ALIMS and Ethics Committee: Submit the CTA and ethics application simultaneously. This approach streamlines the review process, which typically takes up to 60 days, allowing for a more efficient timeline.
Local Representation: Appointing a local representative or a contract research organization (CRO) is essential. This local presence facilitates communication with regulatory bodies and ensures compliance with local laws. It also enhances the understanding of the regulatory landscape and fosters trust with stakeholders.
Review Period: During the review period, both ALIMS and the ethics committee assess the application. Local representation plays a vital role in addressing any queries or concerns that may arise, ensuring a smooth review process.
Addressing Queries: Be prepared to respond promptly to any requests for additional information from regulatory authorities. This responsiveness can significantly impact the timeline of the approval process, making it a critical factor for success.
Final Approval: Upon successful review, formal authorization is granted to initiate the testing phase. This ensures that all conditions set by ALIMS and the ethics committee are met, paving the way for the trial.
The significance of local representation is underscored by Serbia's diverse population of over 7 million, which enhances patient recruitment and representation in studies. Recent statistics indicate that international sponsors account for 84% of ongoing studies in the region, highlighting Serbia's growing status as a center for clinical research. Furthermore, expert insights emphasize that local representation not only simplifies the approval process but also enhances the quality of research data, ensuring compliance with international standards. As Serbia continues to align its regulatory framework with EU standards, the regulatory oversight of gene therapy trials in Serbia becomes increasingly essential for local representatives in navigating the complexities of genetic therapy studies.
Conducting gene therapy trials in Serbia presents several strategic advantages:
The regulatory oversight of gene therapy trials in Serbia is designed for quick approvals, with clinical study protocols frequently authorized within 30 days, and some requiring as few as three weeks. This efficiency significantly decreases the time to begin experiments compared to Western Europe, where schedules can be considerably longer.
Diverse Patient Population: The country features a diverse demographic landscape, with 21.3% of the population over the age of 65 and a significant portion aged between 15 and 64. This diversity allows for the recruitment of patients with a wide range of genetic conditions, enhancing the representativeness and effectiveness of gene therapy trials.
Cost-Effectiveness: Serbia provides moderate research costs and investigator fees, making it a financially appealing choice for sponsors. The cost reductions can be significant compared to Western Europe, enabling a more efficient distribution of resources in medical research.
Skilled Investigators: With a knowledgeable workforce, Serbia features a growing pool of experienced medical researchers and accredited institutions. This expertise guarantees high-quality implementation of studies, adhering to international Good Clinical Practice (GCP) standards.
Supportive Government Policies: The Serbian government actively encourages clinical research through various incentives and support for international collaborations. This proactive stance not only streamlines the testing process but also fosters a conducive environment for innovation and development in the biopharmaceutical sector, highlighting the importance of regulatory oversight of gene therapy trials in Serbia.
Comprehensive Clinical Study Management Services: bioaccess provides a complete range of services including feasibility studies, site selection, compliance reviews, study setup, import permits, project management, and reporting. This comprehensive approach ensures that all aspects of the clinical trial process are managed effectively, driving global health improvement through international collaboration and innovation in Medtech.

The regulatory landscape for gene therapy trials in Serbia is evolving, establishing a robust framework that aligns with EU standards while fostering innovation in medical research. This environment is crucial for ensuring that clinical studies are conducted ethically and efficiently, ultimately promoting advancements in gene therapy that can lead to significant medical breakthroughs.
Key aspects of the regulatory oversight process are essential to understand, including:
The advantages of conducting trials in Serbia-such as quick approval timelines, a diverse patient population, and cost-effectiveness-highlight the country's potential as an emerging hub for gene therapy research.
In light of these insights, stakeholders in the biopharmaceutical sector should consider Serbia as a strategic location for gene therapy trials. By leveraging the supportive regulatory framework and the comprehensive services offered by organizations like bioaccess®, sponsors can navigate the complexities of clinical research effectively. This approach not only enhances the potential for successful outcomes but also contributes to the broader goal of advancing healthcare innovations on a global scale.
What is gene therapy?
Gene therapy is a significant advancement in medical science that involves altering genetic material to address or prevent diseases.
What regulatory framework governs gene therapy trials in Serbia?
The regulatory oversight of gene therapy trials in Serbia is primarily based on the Medicines and Medical Devices Act, which aligns with EU regulations.
Which organizations oversee gene therapy trials in Serbia?
The Agency for Medicines and Medical Devices and local ethics committees provide regulatory oversight for gene therapy trials in Serbia.
What recent changes have been made to the Medicines Act in Serbia?
Recent updates to the Medicines Act aim to streamline regulatory processes, enhance compliance with EU standards, and foster innovation in genetic therapy.
How does Serbia position itself for genetic therapy research by 2025?
Serbia is solidifying its position as a favorable environment for genetic therapy research, supported by successful trials that demonstrate its capacity for advanced therapeutic developments.
What is the focus of the regulatory oversight for gene therapy trials in Serbia?
The regulatory oversight emphasizes safety and efficacy, ensuring that all therapy products undergo rigorous evaluation before entering the market.
How does bioaccess® contribute to gene therapy research in Serbia?
Bioaccess® delivers expedited research study results and ensures swift regulatory approvals, research site activation, patient recruitment, and study data management, facilitating the progression of clinical studies.
What role does bioaccess® play in connecting startups with clinical research sites?
Bioaccess® connects innovative Medtech, Biopharma, and Radiopharma startups with leading clinical research sites in the region, enhancing the effectiveness of advancing clinical studies and securing funding.